Testing conditions for CoMo HDS catalyst in the kinetic region: integrated approach using the math calculations and catalytic experiments
Abstract
Keywords
Full Text:
PDFReferences
Wang TE, Yang F, Song M, Han D. Recent advances in the unsupported catalysts for the hydrodesulfurization of fuel. Fuel Proc Technol.2022:235. doi:10.1016/j.fuproc.2022.107386
Ancheyta J. Modeling and simulation of catalytic reactors for petroleum refining. Model Simul Catal Reac Petroleum Refining. 2011. doi:10.1002/9780470933565.fmatter
Kaluža L, Gulková D, Šolcová O, Žilková N, Čejka J. Hydrotreating catalysts supported on organized mesoporous alumina: Optimization of Mo deposition and promotional effects of Co and Ni. Appl Catal A Gen. 2008;351(1):93–101. doi:10.1016/j.apcata.2008.09.002
Huirache-Acuña R, Navarro Yerga RM, Pawelec B. Hydrodesulfurization on Supported CoMoS2 Catalysts Ex Ammonium Tetrathiomolybdate: Effects of Support Morphology and Al Modification Method. Top Catal. 2022;65:1394–1407. doi:10.1007/s11244-022-01647-w
Iqrash Shafiq, Sumeer Shafique, Parveen Akhter, et al. Recent developments in alumina supported hydrodesulfurization catalysts for the production of sulfur-free refinery products: A technical review. Catal Rev. 2020;64:1–86. doi:10.1080/01614940.2020.1780824
Klimov OV, Vatutina YV, Nadeina KA. CoMoB/Al2O3 catalysts for hydrotreating of diesel fuel. The effect of the way of the boron addition to a support or an impregnating solution. Catal Today. 2018;305:192–202. doi:10.1016/j.cattod.2017.07.004
Zhang C, Zhang Y, Zheng H. Improving both the activity and selectivity of CoMo/δ-Al2O3 by phosphorous modification for the hydrodesulfurization of fluid catalytic cracking naphtha. Energy Fuels. 2022;36(7):3825–3834. doi:10.1021/acs.energyfuels.1c04164
Chen Z, Liu Y, Chen J, Zhao Y, et al. Synthesis of alumina-nitrogen-doped carbon support for CoMo catalysts in hydrodesulfurization process. Chin J Chem Engin. 2022;41:392–402. doi:10.1016/j.cjche.2021.09.015
Catita L, Quoineaud AA, Moreaud M, Espinat D, Pichon C, Delpoux O. Impact of citric acid on the impregnation of CoMoP/γ-Al2O3 catalysts: time and spatially resolved MRI and Raman imaging study. Top Catal. 2018;61(14):1474–1484. doi:10.1007/s11244-018-1038-7
Sun J, Mu C, Li Y, Zhao Y, Wang S, Ma X. The hydrotreatment of n-C16 over Pt/HPMo/SBA-15 and the investigation of diffusion effect using a novel W-P criterion. AIChE J. 2021;67(9):e17330. doi:10.1002/aic.17330
Chen A Cheng, Chen SL, Hua D run, et al. Diffusion of heavy oil in well-defined and uniform pore-structure catalyst under hydrodemetallization reaction conditions. Chem Eng J. 2013;231:420–426. doi:10.1016/j.cej.2013.07.035
Perego C, Peratello S. Experimental methods in catalytic kinetics. Catal Today. 1999;52(2–3):133–145. doi:10.1016/S0920-5861(99)00071-1
Dautzenberg FM. Ten guidelines for catalyst testing. ACS Symposium Ser. 1989:99–119. doi:10.1021/bk-1989-0411.ch011
Chen J, Yang H, Ring Z. Study of intra-particle diffusion effect on hydrodesulphurization of dibenzothiophenic compounds. Catal Today. 2005;109(1):93–98. doi:10.1016/j.cattod.2005.08.006
PA Ramachandran RC. Three-phase catalytic reactors. Gordon Breach Sci Pub. 1983.
Marroquín G, Ancheyta J, Esteban C. A batch reactor study to determine effectiveness factors of commercial HDS catalyst. Catal Today. 2005;104(1):70–75. doi:10.1016/J.CATTOD.2005.03.026
Chen J, Mulgundmath V, Wang N. Accounting for vapor-liquid equilibrium in the modeling and simulation of a commercial hydrotreating reactor. Ind Eng Chem Res. 2011;50(3):1571–1579. doi:10.1021/ie101550g
Bhaskar M, Valavarasu G, Sairam B, Balaraman KS, Balu K. Three-phase reactor model to simulate the performance of pilot-plant and industrial trickle-bed reactors sustaining hydrotreating reactions. Ind Eng Chem Res. 2004;43(21):6654–6669. doi:10.1021/ie049642b
Palos R, Gutiérrez A, Hita I, et al. Kinetic modeling of hydrotreating for enhanced upgrading of light cycle oil. Ind Eng Chem Res. 2019;58(29):13064–13075. doi:10.1021/acs.iecr.9b02095
Alvarez-Majmutov A, Chen J. Modeling and simulation of a multibed industrial hydrotreater with vapor-liquid equilibrium. Ind Eng Chem Res. 2014;53(26):10566–10575. doi:10.1021/ie501032j
Mijatović IM, Glisic SB, Orlović AM. Modeling a catalytic reactor for hydrotreating of straight-run gas oil blended with fluid catalytic cracking naphtha and light cycle oil: influence of vapor–liquid equilibrium. Ind Eng Chem Res. 2014;53(49):19104–19116. doi:10.1021/ie503188p
Jarullah AT, Mujtaba IM, Wood AS. Kinetic model development and simulation of simultaneous hydrodenitrogenation and hydrodemetallization of crude oil in trickle bed reactor. Fuel. 2011;90(6):2165–2181. doi:10.1016/j.fuel.2011.01.025
Macías MJ, Ancheyta J. Simulation of an isothermal hydrodesulfurization small reactor with different catalyst particle shapes. Catal Today. 2004;98(1):243–252. doi:10.1016/j.cattod.2004.07.038
Mederos FS, Ancheyta J, Elizalde I. Dynamic modeling and simulation of hydrotreating of gas oil obtained from heavy crude oil. Appl Catal A Gen. 2012;425–426:13–27. doi:10.1016/j.apcata.2012.02.034
da Rocha Novaes L, de Resende NS, Salim VMM, Secchi AR. Modeling, simulation and kinetic parameter estimation for diesel hydrotreating. Fuel. 2017;209:184–193. doi:10.1016/j.fuel.2017.07.092
Korsten H, Hoffmann U. Three-phase reactor model for hydrotreating in pilot trickle-bed reactors. AIChE J. 1996;42(5):1350–1360. doi:10.1002/aic.690420515
Shokri S, Zarrinpashne S. A mathematical model for calculation of effectiveness factor in catalyst pellets of hydrotreating process. Pet Coal. 2006;48(1):27–33.
Nadeina KA, Danilevich VV, Kazakov MO. Silicon doping effect on the properties of the hydrotreating catalysts of FCC feedstock pretreatment. Appl Catal B Environ. 2021;280:119415. doi:10.1016/j.apcatb.2020.119415
Vatutina YV, Kazakov MO, Nadeina KA. Is it possible to reactivate hydrotreating catalyst poisoned by silicon? Catal Today. 2021;378:43–56. doi:10.1016/j.cattod.2021.03.005
Fogler SH. Essentials of chemical reaction engineering: essenti chemica reactio engi. Pearson Education; 2010.
Mederos FS, Ancheyta J, Chen J. Review on criteria to ensure ideal behaviors in trickle-bed reactors. Appl Catal A Gen. 2009;355(1):1–19. doi:10.1016/j.apcata.2008.11.018
Rodríguez MA, Ancheyta J. Modeling of hydrodesulfurization (HDS), hydrodenitrogenation (HDN), and the hydrogenation of aromatics (HDA) in a vacuum gas oil hydrotreater. Energy Fuels. 2004;18(3):789–794. doi:10.1021/ef030172s
Felder R. Catalytic reactor design, by M. Orhan tarhan. McGraw‐Hill, 1983. В: Aiche J. 1984:372. doi:10.1002/aic.690300127
Mik IA, Klenov OP, Kazakov MO, Nadeina KA, Klimov O V, Noskov AS. Optimization of grading guard systems for trapping of particulates to prevent pressure drop buildup in gas oil hydrotreater. Fuel. 2021;285:119149. doi:10.1016/j.fuel.2020.119149
Ahmed T. Hydrocarbon Phase Behaviour . Gulf, Houston, TX. 1989:226.
Biardi G, Baldi G. Three-phase catalytic reactors. Catal Today. 1999;52(2):223–234. doi:10.1016/S0920-5861(99)00077-2
Ancheyta J, Angeles MJ, Macías MJ, Marroquín G, Morales R. Changes in apparent reaction order and activation energy in the hydrodesulfurization of real feedstocks. Energy Fuels. 2002;16(1):189–193. doi:10.1021/ef0101917
Ancheyta-Juárez J, Aguilar-Rodríguez E, Salazar-Sotelo D, Betancourt-Rivera G, Leiva-Nuncio M. Hydrotreating of straight run gas oil-light cycle oil blends. Appl Catal A Gen. 1999;180(1–2):195–205. doi:10.1016/S0926-860X(98)00351-2
Macías Hernández MJ, Morales RD, Ramírez-Lopez A. Simulation of the effectiveness factor for a tri-lobular catalyst on the hydrodesulfurization of diesel. 2009;7(1). doi:10.2202/1542-6580.1806
Duduković MP, Larachi F, Mills PL. Multiphase catalytic reactors: a perspective on current knowledge and future trends. Catal Rev. 2002;44(1):123–246. doi:10.1081/CR-120001460
Satterfield CN. Trickle-bed reactors. AlChE J. 1975;21(2):209–228. doi:10.1002/aic.690210202
Li C, Chen YW, Tsai MC. Highly restrictive diffusion under hydrotreating reactions of heavy residue oils. Ind Eng Chem Res. 1995;34(3):898–905. doi:10.1021/ie00042a024
Scamangas A, Papayannakos N, Marangozis J. Catalytic hydrodesulfurization of a petroleum residue. Chem Eng Sci. 1982;37(12):1810–1812. doi:10.1016/0009-2509(82)80053-5
G. F. Froment, K.B. Bischoff J de W. Chemical reactor analysis and design. 1990.
Chang J, Liu J, Li D. Kinetics of resid hydrotreating reactions. Catal Today. 1998;43(3–4):233–239. doi:10.1016/S0920-5861(98)00152-7
Aris R. The Mathematical Theory of Diffusion and Reaction in Permeable Catalysts: The theory of the steady state. Oxford Uni.; 1975.
Carberry JJ. Chemical and Catalytic Reaction Engineering. Courier Corporation; 2001.
Thommes M, Kaneko K, Neimark A V., et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem. 2015;87(9–10):1051–1069. doi:10.1515/pac-2014-1117
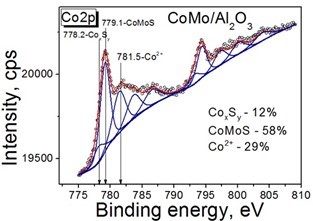
Wang HW, Skeldon P, Thompson GE. XPS studies of MoS2 formation from ammonium tetrathiomolybdate solutions. Surf Coatings Technol. 1997;91:200–207. doi:10.1016/S0257-8972(96)03186-6
Gandubert AD, Legens C, Guillaume D, Payen E. X-ray photoelectron spectroscopy surface quantification of sulfided CoMoP catalysts – relation between activity and promoted sites – Part I: influence of the Co/Mo Ratio. Surf Interface Anal. 2006;38:206–209. doi:10.1002/sia.2249
Vatutina Y V, Klimov O V, Stolyarova EA, et al. Influence of the phosphorus addition ways on properties of CoMo-catalysts of hydrotreating. Catal Today. 2019;329:13–23. doi:10.1016/j.cattod.2019.01.005
Pecoraro T.A., Chianelli R.R. Hydrodesulfurization catalysis by transition metal sulfides. J Catal. 1981;67:430. doi:10.1016/0021-9517(81)90303-1
DOI: https://doi.org/10.15826/chimtech.2023.10.2.08
Copyright (c) 2023 Polina P. Mukhacheva, Yuliya V. Vatutina, Ivan A. Mik, Ksenia A. Nadeina, Maxim O. Kazakov, Oleg P. Klenov, Oleg V. Klimov, Aleksandr S. Noskov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







