Kinetics of solid-state oxidation of iron, copper and zinc sulfide mixture
Abstract
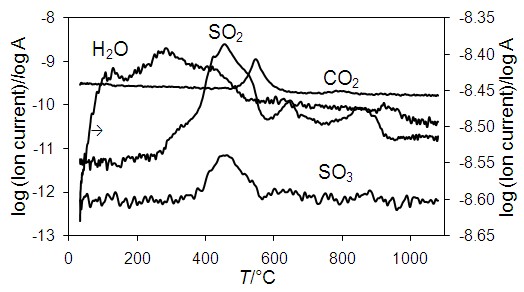
The kinetics of solid-state oxidation by air of iron, copper and zinc sulfide natural mixture, which is typical of the pyritic copper ores, is investigated. Using the high-temperature X-ray powder diffraction, thermogravimetry and differential scanning calorimetry, it was found that the process can be represented by five exothermic elementary reactions, corresponding to intensive burning of iron, copper and zinc sulfides, and two endothermic ones, associated with decomposition of copper and iron sulfates. Kinetic analysis is performed by Kissinger and Augis–Bennett methods, the model-free function mechanism was determined from y(α) master plots and iterative optimization of the kinetic parameters. The limiting steps of these reactions are nucleation and crystal growth, and the values of activation energy, pre-exponential factor and Avrami exponent are in the ranges of 140–459 kJ·mol–1, 1.41·104–3.49·1031 s–1, and 1.0–1.7, respectively. Crystallization is followed by an increase in the number of nuclei, which may be formed both at the interface and in the bulk of the ore particles, and crystal growth is one-dimensional and controlled by a chemical reaction at the phase boundary or diffusion. The results of the work can contribute to the development of theoretical ideas about the physicochemical transformations of pyritic ores and concentrates during pyrometallurgical operations.
Keywords
Full Text:
PDFReferences
Schlesinger ME, King MJ, Sole KC, Davenport WG. Extractive Metallurgy of Copper. 5th Edition. Oxford: Elsevier; 2011.
Selivanov EN, Gulyaeva RI, Klyushnikov AM. Study of structure and phase composition of copper-cobalt sulfide ores of Dergamyshskoe deposit. Tsvetnye Metally. 2016;3:13–17. doi:10.17580/tsm.2016.03.02
Melekestseva IYu, Maslennikov VV, Maslennikova SP. Trace elements in sulfides of the Dergamysh cobalt-bearing massive sulfide deposit, the Southern Urals: Mode of occurrence and matter sources. Lithosphere (Russia). 2020;20(4):499–516. doi:10.24930/1681-9004-2020-20-4-499-516
Nagaeva SP, Mezentseva OP, Kozorez MV. Mineralogical researches of copper cobalt-containing ores of Dergamysh deposit. Gornyi Zhurnal/Mining J. 2014;11:31–4.
Cusano G, Gonzalo MR, Farrell F, Remus R, Roudier S, Delgado Sancho L. Best Available Techniques (BAT) Reference Document for the main Non-Ferrous Metals Industries, EUR 28648 EN, 902–910.
Reznik ID, Sobol SI, Khudyakov VM. Cobalt. Vol. 1. Moscow: Mashinostroyenie; 1995 (In Russian).
Crundwell FK, Moats MS, Ramachandran V, Robinson TG, Dawenport WG. Extractive metallurgy of nickel, cobalt and platinum-group metals. Oxford: Elsevier; 2011. doi:10.1016/B978-0-08-096809-4.10038-3
Warner AEM, Diaz CM, Dalvi AD, Mackey PJ, Tarasov AV, Jones RT. JOM world nonferrous smelter survey part IV: nickel: sulfide. JOM. 2007;59:58–72. doi:10.1007/s11837-007-0056-x
Selivanov EN, Klyushnikov AM, Gulyaeva RI. Use of quartz-containing materials as fluxes in copper smelting production. Metallurgist. 2017;61(1–2):155–161. doi:10.1007/s11015-017-0469-x
Selivanov EN, Klyushnikov AM, Gulyaeva RI. Application of sulfide copper ores oxidizing roasting products as sulfidizing agent during melting nickel raw materials to matte. Metallurgist. 2019;63(7–8):867–887. doi:10.1007/s11015–019–00901–z
Klyushnikov AM, Gulyaeva RI, Selivanov EN, Pikalov SM. Kinetics and mechanism of oxidation for nickel-containing pyrrhotite tailings. Int J Miner Metall Mater. 2021;28(9):1469–1477. doi:10.1007/s12613-020-2109-x
Klyushnikov A, Gulyaeva R, Pikalov S. Cold crystallization kinetics of slag from the joint smelting of oxidized nickel and sulfide copper ores. J Therm Anal Calorim. 2022;147:12165–12176. doi:10.1007/s10973-022-11429-x
Klyushnikov AM. Modeling of exchange interactions in melts formed during joint smelting of oxidized nickel ores and pyrrhotite concentrates. Metallurgist. 2022;66(1–2):190–199. doi:10.1007/s11015-022-01314-1
Božinović K, Štrbac N, Mitovski A, Sokić M, Minić D, Marković B, Stojanović J. Thermal decomposition and kinetics of pentlandite-bearing ore oxidation in the air atmosphere. Metals. 2021;11:1364. doi:10.3390/met11091364
Smirnov VI, Tikhonov AI. Obzhig mednyh rud i koncentratov (teoriya i praktika) (Roasting of Copper Ores and Concentrates (Theory and Practice)). Moscow: Metallurgiya; 1966 (In Russian).
Devia M, Wilkomirsky I, Parra R. Roasting kinetics of high-arsenic copper concentrates: a review. Miner Metall Process. 2012;29(2):121–128. doi:10.1007/BF03402403
Dimitrov R, Boyanov B. Investigation of the oxidation of metal sulphides and sulphide concentrates Thermochim Acta. 1983;64:27–37. doi:10.1016/0040-6031(83)80125-7
Hua Yixin, Cai Chaojun, Cui Yan. Microwave-enhanced roasting of copper sulfide concentrate in the presence of CaCO3. Sep Purif Technol. 2006;50:22–29. doi:10.1016/j.seppur.2005.11.003
Mitovski A, Strbac N, Mihajlovic I, Sokić M, Stojanović J. Thermodynamic and kinetic analysis of the polymetallic copper concentrate oxidation process. J Therm Anal Calorim. 2014;118:1277–1285. doi:10.1007/s10973-014-3838-8
Prasad S, Pandey BD. Thermoanalytical studies on copper-iron sulphides. J Therm Anal Calorim. 1999;58:625–637. doi:10.1023/A:1010108729034
Prasad PN, Lennartsson A, Samuelsson C. A mineralogical investigation of sintering in Cu-rich polymetallic concentrates during roasting in inert atmosphere. Metall Mater Trans B. 2020;51:1446–1459. doi:10.1007/s11663-020-01850-8
Shamsuddin M, Sohn HY. Constitutive topics in physical chemistry of high-temperature nonferrous metallurgy – a review: part 1. Sulfide roasting and smelting. JOM. 2019;71(9):3253–3265. doi:10.1007/s11837-019-03620-7
Souza R, Queiroz C, Brant J, Brocchi E. Pyrometallurgical processing of a low copper content concentrate based on a thermodynamic assessment. Miner Eng. 2019;130:156–164. doi:10.1016/j.mineng.2018.10.015
Wan X, Shi J, Taskinen P, Jokilaakso A. Extraction of copper from copper-bearing materials by sulfation roasting with SO2–O2 gas. JOM. 2020;72:3436–3446. doi:10.1007/s11837-020-04300-7
Wilkomirsky I, Parra R, Parada F, Balladares E, Seguel E, Etcheverry J, Díaz R. Thermodynamic and kinetic mechanisms of bornite/chalcopyrite/magnetite formation during partial roasting of high-arsenic copper concentrates. Metall Mater Trans B. 2020;51:1540–1551. doi:10.1007/s11663-020-01870-4
Yang F, Wu C, Cui Y, Lu G. Apparent activation energy for spontaneous combustion of sulfide concentrates in storage yard. Trans Nonferrous Metals Soc China. 2011;21(2):395–401. doi:10.1016/S1003-6326(11)60727-9
Živcović Ž, Mitevska N, Savović V. Kinetics and mechanism of the chalcopyrite-pyrite concentrate oxidation process. Thermochim Acta. 1996;282–283:121–130. doi:10.1016/0040-6031(96)02883-3
Chen TT, Dutrizac JE. Mineralogical changes occurring during the fluid-bed roasting of zinc sulfide concentrates. JOM. 2004;56:46–51. doi:10.1007/s11837-004-0235-y
Snurnikov AP. Gidrometallurgiya cinka (Hydrometallurgy of zinc). Moscow: Metallurgiya; 1981 (In Russian).
Dunn JG, Jayaweera SAA. Effect of heating rate on the TG curve during the oxidation of nickel sulphide concentrates. Thermochim Acta. 1983;61:313–317. doi:10.1016/0040-6031(83)80286-X
Yu D, Utigard TA. TG/DTA Study on the Oxidation of Nickel Concentrate. Thermochim Acta. 2012;533:56–65. doi:10.1016/j.tca.2012.01.017
Thoumsin FJ, Coussement R. Fluid-bed roasting reactions of copper and cobalt sulfide concentrates. JOM. 1964;16:831–834. doi:10.1007/BF03378299
Hu G, Dam-Johansen K, Wedel S, Hansen JP. Decomposition and oxidation of pyrite. Prog Energy Combust Sci. 2006;32:295–314. doi:10.1016/J.PECS.2005.11.004
Dunn JG, Mackey LC. The measurement of ignition temperatures and extents of reaction on iron and iron-nickel sulfides. J Therm Anal. 1991;37:2143–2164. doi:10.1007/BF01905584
Luganov VA, Shabalin VI. Thermal dissociation of pyrite during processing of pyrite-containing raw materials. Can Metall Q. 1994;33(3):169–174. doi:10.1179/cmq.1994.33.3.169
Dunn J.G The oxidation of sulphide minerals. Thermochim Acta. 1997;300:127–139. doi:10.1016/S0040-6031(96)03132-2
Eneroth E, Koch CB. Crystallite size of haematite from thermal oxidation of pyrite and marcasite – effects of grain size and iron disulphide polymorph. Miner Eng. 2003;16:1257–1267. doi:10.1016/j.mineng.2003.07.004
Ferrow EA, Mannerstrand M, Sjöberg B. Reaction kinetics and oxidation mechanisms of the conversion of pyrite to ferrous sulphate: a mössbauer spectroscopy study. Hyperfine Interact. 2005;163:10919. doi:10.1007/s10751-005-9200-6
Aylmore MG, Lincoln F. Mechanochemical milling-induced reactions between gases and sulfide minerals. I. Reactions of SO2 with arsenopyrite, pyrrhotite and pyrite. J Alloys Compd. 2000;309:61–74. doi:10.1016/S0925-8388(00)00916-6
Vázquez M, Moreno-Ventas I, Raposo I, Palma A, Díaz MJ. Kinetic of pyrite thermal degradation under oxidative environment. J Therm Anal Calorim. 2020;141:1157–1163. doi:10.1007/s10973-019-09098-4
Ruan S, Wang C, Jie X, Yin F, Zhang Y, Yao Z, Chen Y. Kinetics of pyrite multi-step thermal decomposition in refractory gold sulphide concentrates. J Therm Anal Calorim. 2022;147:3689–3702. doi:10.1007/s10973-021-10761-y
Wang L, Fan BW, He YT, Li P, Yin DQ, Hu YH. Characteristics of minerals and their associations of transformation processes in pyrite at elevated temperatures: an X-ray diffraction study. Ironmaking Steelmaking. 2014;41(2):147–152. doi:10.1179/1743281213Y.0000000113
Xu H, Guo X, Seaman LA, Harrison AJ, Obrey SJ, Page K. Thermal desulfurization of pyrite: An in situ high-T neutron diffraction and DTA–TGA study. J Mater Res. 2019;34:3243–3253. doi:10.1557/jmr.2019.185
Zhang Y, Li Q, Liu X, Xu B, Yang Y, Jiang T. A thermodynamic analysis on the roasting of pyrite. Mineral. 2019;9:220. doi:10.3390/min9040220
Jorgensen FRA, Moyle FJ. Phases formed during the thermal analysis of pyrite in air. J Therm Anal. 1982;25:473–485. doi:10.1007/BF01912973
Aracena Á, Jerez Ó, Colás Ortíz R, Morales JW. Pyrite oxidation kinetics in an oxygen-nitrogen atmosphere at temperatures from 400 to 500 °C. Can Metal Q. 2016;55:195–201. doi:10.1080/00084433.2015.1126904
Reimers GW, Hjelmstad KE. Analysis of the oxidation of chalcopyrite, chalcocite, galena, pyrrhotite, marcasite and arsenopyrite. Department of the Interior, Bureau of Mines. Report of investigations 9118 (United States. Bureau of Mines). Pittsburgh, Pa. U.S.: 1987.
Malek TJ, Chaki SH, Deshpande MP. Structural, morphological, optical, thermal and magnetic study of mackinawite FeS nanoparticles synthesized by wet chemical reduction technique. Physica B: Condensed Matter. 2018;546:59–66. doi:10.1016/j.physb.2018.07.024
Asaki Z, Matsutomo T, Tanabe T, Condo Y. Oxidation of Dense Iron Sulfide. Metall Mater Trans. B. 1983;14:109–116. doi:10.1007/BF02670877
Kennedy T, Sturman BT. The Oxidation of Iron (II) Sulfide. J Therm Anal. 1975;8:329–37. doi:10.1007/BF01904010.
Asaki Z, Condo Y. Oxidation Kinetics of Iron Sulfide in the Form of Dense Plate, Pellet and Single Particle 1989;35:1751–1759. doi:10.1007/BF01911664
Coombs PG, Munir ZA. The mechanism of oxidation of ferrous sulfide (FeS) powders in the range of 648 to 923 K. Metall Mater Trans B. 1989;20:661–667. doi:10.1007/BF02655922
Gulyaeva RI, Selivanov EN, Vershinin AD. Nonisothermal Oxidation of Pyrrhotines. Russian Metallurgy (Metally). 2003;4:299–304.
Alksnis A, Li B, Elliott R, Barati M. Kinetics of Oxidation of Pyrrhotite. In: B. Davis et al., editors. Extraction 2018, The Minerals, Metals & Materials Series. Cham: Springer; pp. 403–413. doi:10.1007/978–3–319–95022–8_32
Habashi F, Dugdale R. The action of sulfur trioxide on chalcopyrite. Metall Mater Trans. B. 1973;4:1553–1556. doi:10.1007/BF02668007
Leung L.S. The overall kinetics of roasting of chalcopyrite. Metall Mater Trans. B. 1975;6:341–343. doi:10.1007/BF02913578
Aneesuddin M, Char PN, Hussain MR, Saxena ER. Studies on thermal oxidation of chalcopyrite from Chitradurga, Karnataka State, India. J Therm Anal. 1983;26:205–215. doi:10.1007/BF01913204
Chaubal PC, Sohn HY. Intrinsic kinetics of the oxidation of chalcopyrite particles under isothermal and nonisothermal conditions. Metall Mater Trans. B. 1986;17:51–60. doi:10.1007/BF02670818
Cocić MB, Logar MM, Cocić SLj, Dević SS, Manasijević DM. Transformation of chalcopyrite in the roasting process of copper concentrate in fluidized bed reactor. JOM. 2011;63:55–59. doi:10.1007/s11837-011-0078-2
Živcović Ž, Štrbać N, Živcović D, Velinovski V, Mihajlović I. Kinetic study and mechanism of chalcocite and covellite oxidation process. J Therm Anal Calorim. 2005;79:715–720. doi:10.1007/s10973-005-0601-1
Ramakrishna Rao VVVNS, Abraham KP. Kinetics of oxidation of copper sulfide. Metall Mater Trans B. 1971;2:2463–2470. doi:10.1007/BF02814883
Dunn JG, Ginting AR, O’Connor B. A thermoanalytical study of the oxidation of chalcocite. J Therm Anal. 1994;41:671–686. doi:10.1007/BF02549341
Benlyamani M, Ajersch F. Agglomeration of particles during roasting of zinc sulfide concentrates. Metall Mater Trans B. 1986;17:647–656. doi:10.1007/BF02657127
Dimitrov R, Bonev I. Mechanism of zinc sulphide oxidation. Thermochim Acta. 1986;106:9–25. doi:10.1016/0040-6031(86)85111-5
Dimitrov RI, Boyanov BS. Oxidation of Metal Sulphides and Determination of Characteristic Temperatures by DTA and TG. J Therm Anal Calorim. 2000;61:181–189. doi:10.1023/A:1010181112713
Graydon JW, Kirk DW. A Microscopic study of the transformation of sphalerite particles during the roasting of zinc concentrate. Metall Mater Trans. B. 1988;19:141–146. doi:10.1007/BF02666500
Gulyaeva RI, Selivanov EN, Pikalov SM. Mechanism and Kinetics of the Thermal Oxidation of Natural Sphalerite. Russian Metallurgy (Metally). 2018;3:221–227. doi:10.1134/S0036029518030047
Natesan K, Philbrook WO. Oxidation kinetic studies of zinc sulfide in a fluidized bed reactor. Metall Mater Trans B. 1970;1:1353–60. doi:10.1007/BF02900254
Marzoughi O, Halali M, Moradkhani D, Pickle CA. Kinetics of Roasting of a Sphalerite Concentrate. In: B. Davis et al., editors. Extraction 2018. The Minerals, Metals & Materials Series. Cham: Springer, 2018. doi:10.1007/978-3-319-95022-8_44
Asaki Z, Nitta M, Tanabe T, Condo Y. Oxidation of cobalt sulfide. Metall Mater Trans B. 1986;17:367–373. doi:10.1007/BF02655084
Boyanov BS. Differential thermal study of the interactions between sulphates, oxides and ferrites. Thermochim Acta. 1997;302:109–115. doi:10.1016/S0040-6031(97)00199-8
Tsukada H, Asaki Z, Tanabe T, Condo Y. Oxidation of mixed copper-iron sulfide. Metall Mater Trans B. 1981;12:603–609. doi:10.1007/BF02654333
Arkhangelsky IV, Dunaev AV, Makarenko IV, Tikhonov NA, Belyaev SS, Tarasov AV. Non-isothermal kinetic methods. Workbook and Laboratory Manual. Edition Open Access. 2013. http://edition-open-access.de/media/textbooks/1/Textbooks1.pdf
Chung FH. A new X-ray diffraction method for quantitative multicomponent analysis. Adv X-Ray Anal. 1973;17:106–115. doi:10.1154/S0376030800005231
Hubbard CR, Evans EH, Smith DK. The reference intensity ratio, I/Ic, for computer simulated powder patterns. J Appl Crystallogr. 1976;9:169–174. doi:10.1107/S0021889876010807
Altomare A, Corriero N, Cuocci C, Falcicchio A, Moliterni A, Rizzi R. QUALX2.0: a qualitative phase analysis software using the freely available database POW_COD. J Appl Crystallogr. 2015;48:598–603. doi:10.1107/S1600576715002319
Arshad MA, Maaroufi AK. Recent advances in kinetics and mechanisms of condensed phase processes: a mini-review. Rev Adv Mater Sci. 2017;51:177–187.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19. doi:10.1016/j.tca.2011.03.034
Henderson DW. Thermal analysis of non-isothermal crystallization kinetics in glass forming liquids. J Non-Cryst Solids. 1979;30:301–315. doi:10.1016/0022-3093(79)90169-8
Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand. 1956;57:217–221. doi:10.6028/jres.057.026
Augis JA., Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal. 1978;13:283–292. doi:10.1007/BF01912301
Pelovski YG, Petkova V. Mechanism and kinetics of inorganic sulphates decomposition. J Therm Anal. 1997;49:1227–1241. doi:10.1007/BF01983679
Horoshavin AG. Forsterite 2MgO·SiO2. Moscow: Teplotekhnika; 2004 (In Russian).
Yamaguchi T, Shiraishi T. Kinetic Studies of Eutectoid Decomposition of CuFe5O8. J Am Ceram Soc. 1971;54:556–558. doi:10.1111/j.1151-2916.1971.tb12206.x
Luo YH, Zhu DQ, Pan J, Zhou XL. Thermal decomposition behaviour and kinetics of Xinjiang siderite ore. Mineral Processing and Extractive Metallurgy. 2016;125:17–25. doi:10.1080/03719553.2015.1118213
Petkova V, Pelovski YG. Comparative DSC study on thermal decomposition of iron sulphates. J Therm Anal Calorim. 2008;93:847–852. doi:10.1007/S10973-008-9302-X
Petkova V, Pelovski YG, Paneva D, Mitov I. Influence of gas media on the thermal decomposition of second valence iron sulphates. J Therm Anal Calorim. 2011;105:793–803. doi:10.1007/S10973-010-1242-6
Choi K, Kim S, Kim M, Park H. Oxidation Behavior of Copper Concentrate, Gold Concentrate, and Their Mixtures Between 1173 K (900 °C) and 1373 K (1100 °C). Metall Mater Trans B. 2019;50:1300–1308. doi:10.1007/s11663-019-01575-3
Matusita K, Sakka S. Kinetic study of crystallization of glass by differential thermal analysis – criterion on application of Kissinger plot. J Non-Cryst Solids. 1980;38–39(2):741–746. doi:10.1016/0022-3093(80)90525-6
DOI: https://doi.org/10.15826/chimtech.2023.10.2.02
Copyright (c) 2023 Alexander M. Klyushnikov, Sergey M. Pikalov, Roza I. Gulyaeva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







