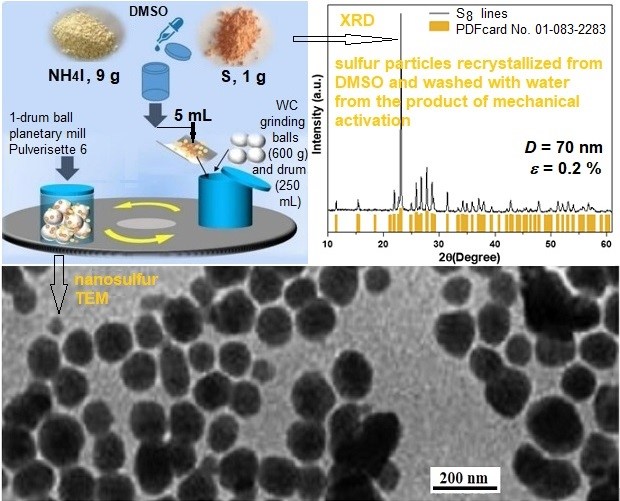
Mechanochemical recrystallization: Forgotten basics and new possibilities
Abstract
Keywords
Full Text:
PDFReferences
Friščić T, Childs SL, Rizvi SAA, Jones W. The role of solvent in mechanochemical and sonochemical cocrystal formation: a solubility-based approach for predicting cocrystallisation outcome. CrystEngComm. 2009;11(3):418–426. doi:10.1039/B815174A
Meenatchi B, Renuga V. Protic ionic liquids assisted synthesis and characterization of sulfur nanoparticles and CdS and ZnS nanomaterials. Chem Sci Trans. 2015;4(2):577–587. doi:10.7598/cst2015.1028
Ying P, Yu J, Su W. Liquid‐assisted grinding mechanochemistry in the synthesis of pharmaceuticals. Adv Synth Catal. 2021;363(5):1246-1271. doi:10.1002/adsc.202001245
Zaikin PA, Dyan OkT, Elanov IR, Borodkin GI. Ionic liquid-assisted grinding: An electrophilic fluorination benchmark. Molecules. 2021;26(19):5756. doi:10.3390/molecules26195756
Kosimov A, Yusibova G, Aruväli J, Paiste P, Käärik M, Leis J, Kikas A, Kisand V, Šmits K, Kongi N. Liquid-assisted grinding/compression: A facile mechanosynthetic route for the production of high-performing Co–N–C electrocatalyst materials. Green Chem. 2022;24(1):305–314. doi:10.1039/D1GC03433B
Loya JD, Li SJ, Unruh DK, Hutchins KM. Mechanochemistry as a tool for crystallizing inaccessible solids from viscous liquid components. Cryst. Growth Des. 2022;22(1):285–292. doi:10.1021/acs.cgd.1c00929
Baláž P, Achimovičová M, Baláž M, Billik P, Cherkezova-Zheleva Z, Criado JM, Delogu F, Dutková E, Gaffet E, Gotor FJ, Kumar R, Mitov I, Rojac T, Senna M, Streletskii A, Wieczorek-Ciurowa K. Hallmarks of mechanochemistry: from nanoparticles to technology. Chem Soc Rev. 2013;42(18):7571–7637. doi:10.1039/C3CS35468G
Boldyreva E. Mechanochemistry of inorganic and organic systems: what is similar, what is different? Chem Soc Rev. 2013;42(18):7719–7738. doi:10.1039/C3CS60052A
Michalchuk AA, Boldyreva EV, Belenguer AM, Emmerling F, Boldyrev VV. Tribochemistry, mechanical alloying, mechanochemistry: what is in a name? Front. Chem. 2021;9(1):685789. doi:10.3389/fchem.2021.685789
Boldyreva EV. Spiers Memorial Lecture: Mechanochemistry, tribochemistry, mechanical alloying – retrospect, achievements and challenges. Faraday Discuss. 2023;241:9–62. doi:10.1039/D2FD00149G
Matsuoka M, Danzuka K. Solid-state recrystallization behavior of binary inorganic salt systems by mechanochemical processing. J Chem Eng Japan. 2009;42(6):393–399. doi:10.1252/jcej.09we068
Katsenis A, Puškarić A, Štrukil V, Mottillo C, Julien PA, Užarević K, Pham M-H, Do T-O, Kimber SAJ, Lazić P, Magdysyuk O, Dinnebier RE, Halasz I, Friščić T. In situ X-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal-organic framework. Nat Commun. 2015;6:6662. doi:10.1038/ncomms7662
Urakaev FKh, Khan NV, Shalabaev ZhS, Tatykaev BB, Nadirov RK, Burkitbaev MM. Synthesis and photocatalytic properties of silver chloride/silver composite colloidal particles. Colloid J. 2020;82(1):76–80. doi:10.1134/S1061933X20010160
Nieto-Castro D, Garcés-Pineda FA, Moneo-Corcuera A, Pato-Doldan B, Gispert-Guirado F, Benet-Buchholz J, Galán-Mascarós JR. Effect of mechanochemical recrystallization on the thermal hysteresis of 1D FeII-triazole spin crossover polymers. Inorg Chem. 2020;59(12):7953–7959. doi:10.1021/acs.inorgchem.9b03284
Kadja GTM, Suprianti TR, Ilmi MM, Khalil M, Mukti RR, Subagjo. Sequential mechanochemical and recrystallization methods for synthesizing hierarchically porous ZSM-5 zeolites. Microporous Mesoporous Mater. 2020;308:110550. doi:10.1016/j.micromeso.2020.110550
Zyryanov VV, Petrov SA, Ulihin AS. Mechanically activated synthesis, characterization and conducting properties of complex perovskites for Ag-based metal-matrix nanocomposites. Ceram Int. 2021;47(20):29499–29503. doi:10.1016/j.ceramint.2021.07.118
Zyryanov VV. Mechanically assisted chemical interaction of doped bismuth oxide with silver. Solid State Ionics. 2022;383:115987. doi:10.1016/j.ssi.2022.115987
Dubadi R, Huang SD, Jaroniec M. Mechanochemical synthesis of nanoparticles for potential antimicrobial applications. Mater. 2023;16(4):1460. doi:10.3390/ma16041460
Burkitbayev MM, Urakaev FKh. Temperature dependence of sulfur solubility in dimethyl sulfoxide and changes in concentration of supersaturated sulfur solutions at 25 degrees C. J Mol Liq. 2020;316:113886. doi:10.1016/j.molliq.2020.113886
Du G-X, Xue Q, Ding H, Li Z. Mechanochemical effects of ZnO powder in a wet super-fine grinding system as indicated by instrumental characterization. Int J Min Process. 2015;141:15–19. doi:10.1016/j.minpro.2015.06.008
Lu J, Lu Z, Li X, Xu H, Li X. Recycling of shell wastes into nanosized calcium carbonate powders with different phase compositions. J Clean Prod. 2015;92:223–229. doi:10.1016/j.jclepro.2014.12.093
Lu J, Cong X, Li Y, Hao Y, Wang C. Scalable recycling of oyster shells into high purity calcite powders by the mechanochemical and hydrothermal treatments. J Clean Prod. 2018;172:1978–1985. doi:10.1016/j.jclepro.2017.11.228
Kurniawan T, Muraza O, Hakeem AS, Al-Amer AM. Mechanochemical route and recrystallization strategy to fabricate mordenite nanoparticles from natural zeolites. Cryst Growth Des. 2017;17(6):3313–3320. doi:10.1021/acs.cgd.7b00295
de Oliveira Y.S., Oliveira A.C., Ayala A.P. Mechanochemically induced solid state transformations: The case of raloxifene hydrochloride. Eur J Pharm Sci. 2018;114:146–154. doi:10.1016/j.ejps.2017.11.028
Yang P, Li X, Li Z, Fang X, Zhang K, Zhuang W, Wu J, Zhu C, Ying H. Green mechanochemical strategy for the construction of a new bio-based nylon 524T ternary salt. ACS Sustain Chem Eng. 2022;10(11):3513–3520. doi:10.1021/acssuschemeng.1c07869
Urakaev FKh, Bulavchenko AI, Uralbekov BM, Massalimov IA, Tatykaev BB, Bolatov AK, Zharlykasimova DN, Burkitbayev MM. Mechanochemical synthesis of colloidal sulphur particles in the Na2S2O3−H2(C4H4O4)−Na2SO3 system. Colloid J. 2016;78(2):210–219. doi:10.1134/S1061933X16020150
Shalabayev Zh, Baláž M, Daneu N, Dutkova E, Bujňáková Z, Kaňuchová M, Dankova Z, Balážová Ľ, Tkáčiková Ľ, Urakaev F, Burkitbayev M. Sulfur-mediated mechanochemical synthesis of spherical and needle-like copper sulfide nanocrystals with antibacterial activity. ACS Sustain Chem Eng. 2019;7(15):12897–12909. doi:10.1021/acssuschemeng.9b01849
Shalabaev ZS, Urakaev FK, Baláž M, Khan NV, Burkitbaev MM. Method for obtaining needle-like copper sulfide (II) nanocrystals. Patent of the Republic of Kazakhstan for utility model No. 5287. Bulletin number: 32. Bulletin date: 14.08.2020. https://gosreestr.kazpatent.kz/Utilitymodel/DownLoadFilePdf?patentId=326616
Khan N, Baláž M, Burkitbayev M, Tatykayev B, Shalabayev Z, Nemakayeva R, Jumagaziyeva A, Niyazbayeva A, Rakhimbek I, Beldeubayev A, Urakaev F. DMSO- mediated solvothermal synthesis of S/AgX (X = Cl, Br) microstructures and study of their photocatalytic and biological activity. Appl Surf Sci. 2022;601:154122. doi:10.1016/j.apsusc.2022.154122
Khan NV, Baláž M, Burkitbayev MM, Tatykayev BB, Shalabayev ZhS., Niyazbayeva AI, Urakaev FKh. Solvothermal DMSO-mediated synthesis of the S/AgI microstructures and their testing as photocatalysts and biological agents. Int J Biol Chem. 2022;15(1):79–89. doi:10.26577/ijbch.2022.v15.i1.09
Urakaev FKh. Mechanochemical synthesis of nanoparticles by a dilution method: determination of the particle mixing coefficient in a ball mill. Mendeleev Commun. 2012;22(4):215–217. doi:10.1016/j.mencom.2012.06.016
Urakaev FK, Burkitbaev MM, Uralbekov BM, Shalabaev ZS. Method for producing sulfur nanoparticles from solutions in dimethyl sulphoxide, using solution of sulfur in dimethyl sulfoxide saturated at room temperature with specific sulfur concentration when diluted with water or acetone. Patent EA33075-B1. Publ. 30 Aug 2019. Derwent 2019-85527S.
Urakaev FKh, Burkitbayev MM, Khan NV. Biological activity of sulfur nanoparticles in the sulfur−dimethyl sulfoxide−water system. Int J Biol Chem. 2022;15(2):54–75. doi:10.26577/ijbch.2022.v15.i2.09
Burkitbaev MM, Khan NV, Madikasimova MS, Oskenbai AK, Urakaev FKh. Method for obtaining sulfur-containing nanocomposites. Patent of the Republic of Kazakhstan for utility model No. 5241. Bulletin number: 30. Bulletin date: 30.07.2020. https://gosreestr.kazpatent.kz/Utilitymodel/DownLoadFilePdf?patentId=325175
Urakaev FKh. Preparation of NaIn(WO4)(2) nanocrystals and a charge for crystal growth via the free-of-rubbing mechanical activation of the Na2CO3–In2O3–WO3 system. Mendeleev Commun. 2016;26(6):546–548. doi:10.1016/j.mencom.2016.11.030
LeBel RG, Goring DAI. Density, viscosity, refractive index, and hygroscopicity of mixtures of water and dimethyl sulfoxide. J Chem Eng Data. 1962;7(1):100–101. doi:10.1021/je60012a032
Ellson R, Stearns R, Mutz M, C Brown C, Browning B, Harris D, Qureshi S, Shieh J, Wold D. In situ DMSO hydration measurements of HTS compound libraries. Comb Chem High Throughput Screen. 2005;8(6):489–498. doi:10.2174/1386207054867382
Waybright TJ, Britt JR, McCloud TG. Overcoming problems of compound storage in DMSO: solvent and process alternatives. J Biomol Screen. 2009;14(6):708–715. doi:10.1177/1087057109335670
Rabiei M, Palevicius A, Dashti A, Nasiri S, Monshi A, Doustmohammadi A, Vilkauskas A, Janusas G. X-ray diffraction analysis and Williamson-Hall method in USDM model for estimating more accurate values of Stress-Strain of unit cell and super cells (2 × 2 × 2) of hydroxyapatite, confirmed by Ultrasonic Pulse-Echo Test. Mater (Basel). 2021;14(11):2949. doi:10.3390/ma14112949
Himabindu B, Latha Devi NSMP, Rajini Kanth B. Microstructural parameters from X-ray peak profile analysis by Williamson-Hall models; A review. Mater Today Proceed. 2021;47(14):4891–4896. doi:10.1016/j.matpr.2021.06.256
Tirpude MP, Tayade NT. Frustrate microstructures composed PbS cluster’s size perspective from XRD by variant models of Williamson-Hall plot method. Preprint. 2022;25:36. doi:10.21203/rs.3.rs-1586320/v1
Nims C, Cron B, Wetherington M, Macalady J, Cosmidis J. Low frequency Raman spectroscopy for micron-scale and in vivo characterization of elemental sulfur in microbial samples. Sci Rep-UK. 2019;9(1):7971. doi:10.1038/s41598-019-44353-6
Assis M, Groppo Filho FC., Pimentel DS., Robeldo T, Gouveia AF, Castro TFD, Fukushima HCS, de Foggi CC, da Costa JPC, Borra RC, Andrés J, Longo E. Ag nanoparticles / AgX (X= Cl, Br, I) composites with enhanced photocatalytic activity and low toxicological effects. Chem Sel. 2020;5(15):4655–4673. doi:10.1002/slct.202000502
DOI: https://doi.org/10.15826/chimtech.2023.10.2.13
Copyright (c) 2023 Farit Kh. Urakaev, Natalya V. Khan, Almagul I. Niyazbayeva, Dinar N. Zharlykasimova, Mukhambetkali M. Burkitbayev

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







