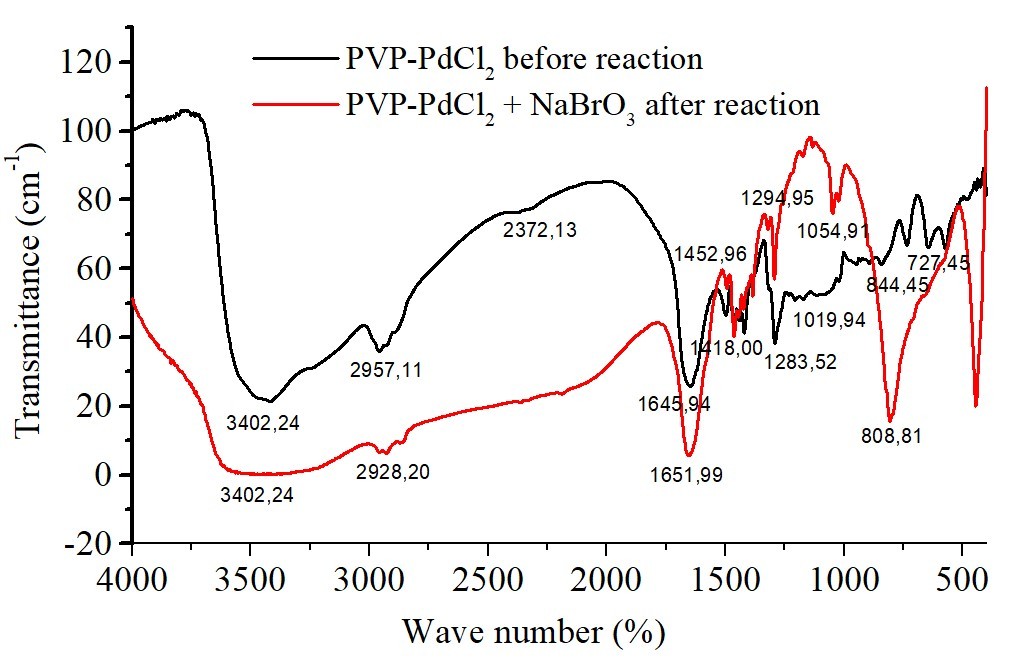
Complexes of polyvinylpyrrolidone and polyethylene glycol with palladium(II) ions: characterization and catalytic activity
Abstract
Keywords
Full Text:
PDFReferences
Weissermel K, Arpe HJ. Industrial Organic Chemistry. 4th ed. Weinheim: Wiley-VCH; 2003. 491 p. doi:10.1002/9783527619191
Tsuji J. Synthetic applications of the palladium-catalyzed oxidation of olefins to ketones. Synth. 1984;369–384. doi:10.1055/s-1984-30848
Takacs JM, Jiang XT. The Wacker reaction and related alkene oxidation reaction. Curr Org Chem. 2003;7(4):369–396. doi:10.2174/1385272033372851
Sharma GVM, Krishna PR. Carbohydrates: from ‘chirons’ to new glycosubstances. Curr Org Chem. 2004;8(13):1187–1209. doi:10.2174/1385272043370096
Kulkarni MG, Shaikh BY, Borhade AS, Chavhan SW, Dhondge AP, Gaikwad DD, Desai MP, Birhade DR, Dhatrak NR. Greening the Wacker process. Tetrahedron Lett. 2013;54:2293–2295. doi:10.1016/j.tetlet.2013.01.082
Pires MJD, Purificação SI, Santos AS, Marques MMB. The role of PEG on Pd- and Cu-catalyzed cross-coupling reactions. Synth. 2017;49:2337–2350. doi:10.1055/s-0036-1589498
Schroeter F, Soellner J, Strassner T. Cyclometalated palladium NHC complexes bearing PEG chains for Suzuki–Miyaura cross-coupling in water. Organometal. 2018;37(22):4267–4275. doi:10.1021/acs.organomet.8b00607
Han GH, Lee SH, Seo M, Lee KY. Effect of polyvinylpyrrolidone (PVP) on palladium catalysts for direct synthesis of hydrogen peroxide from hydrogen and oxygen. RSC Adv. 2020;10:19952–19960. doi:10.1039/D0RA03148H
Lu J. Sodium bromate: an eco-friendly brominating and oxidizing reagent. SYNLETT. 2011;6:0879–0880. doi:10.1055/s-0030-1259755
Bierenstiel M, D’Hondt PJ, Schlaf M. Investigations into the selective oxidation of vicinal diols to a-hydroxy ketones with the NaBrO3/NaHSO3 reagent: pH dependence, stoichiometry, substrates and origin of selectivity. Tetrahedron. 2005;61:4911–4917. doi:10.1016/j.tet.2005.03.056
Yamato T, Shinoda N. Highly efficient oxidation of alcohols catalysed by Nafion-cerium(IV) or Nafion-chromium(III). J Chem Research (S). 2002;8:400–402. doi:10.3184/030823402103172464
Shaabani A, Farhangi E, Rahmati A. Ionic liquid promoted selective oxidation of organic compounds with NaBrO3. Monatsh Chem. 2008;139:905–908. doi:10.1007/s00706-007-0840-x
Al-Hashimi M, Fisset E, Sullivan AC, Wilson JRH. Selective oxidation of sulfides to sulfoxides using a silica immobilised vanadyl alkyl phosphonate catalyst. Tetrahedron Lett.2006;47(46):8017–8019. doi:10.1016/j.tetlet.2006.09.065
Memarian HR, Mohammadpoor-Baltork I, Sadeghi MM, Samani ZS. Potassium peroxodisulfate: a convenient oxidizing agent for aromatization of 1,4-dihydroperidines. Indian J Chem. 2001;40B:727–728. doi:10.1002/chin.200149102
Safari A, Zeynizadeh B. Activated charcoal: a highly efficient promoter for selective oxidation of alcohols to aldehydes and ketones by K2S2O8 at solvent−free conditions. Curr Chem Lett. 2015;4:111–118. doi:10.5267/j.ccl.2015.4.001
Yang XY, Wang R, Wang L, Li J, Mao S, Zhang SQ, Chen N. K2S2O8-promoted C–Se bond formation to construct α-phenylseleno carbonyl compounds and α,β-unsaturated carbonyl compounds. RSC Adv. 2020;10:28902–28905. doi:10.1039/d0ra05927g
Walker FS, Bhattacharya SN, Senoff CV. Alkylated polyamine complexes of palladium(II). Inorg Synth. 1982;21:129–130. doi:10.1002/9780470132524.ch30
McCombs JR, Michel BW, Sigman MS. Catalyst-controlled Wacker-type oxidation of homoallylic alcohols in the absence of protecting groups. J Org Chem. 2011;76(9):3609–3613. doi:10.1021/jo200462a
Liu M, Yan X, Liu H, Yu W. An investigation of the interaction between polyvinylpyrrolidone and metal cations. React Funct Polym. 2000;44(1):55–64. doi:10.1016/S1381-5148(99)00077-2
Gupta MN, Batra R, Tyagi R, Sharma A. Polarity index: the guiding solvent parameter for enzyme stability in aqueous-organic cosolvent mixtures. Biotechnol Prog. 1997;13(3):284–288. doi:10.1021/bp9700263
Moiseyev II. Pi-kompleksy v zhidkofaznom okislenii olefinov [Pi-Complexes in liquid-phase oxidation of olefins]. Moscow: Nauka; 1970. 242 p. Russian.
Huheey JE. Inorganic Chemistry: Principles of structure and reactivity. 3d ed. New-York: Harper and Row; 1983. 936 р.
Rokosz K, Hryniewicz T, Matysek D, Raaen S, Valicek J, Dudek L, Harnicarova M. SEM, EDS and XPS analysis of the coatings obtained on titanium after plasma electrolytic oxidation in electrolytes containing copper nitrate. Mater. 2016;9(5):318. doi:10.3390/ma9050318
Akbayeva DN, Bakirova BS, Seilkhanova GA, Sitzmann H. Synthesis, characterization, and catalytic activity of palladium-polyvinylpyrrolidone complex in oxidation of octene-1. Bull Chem React Eng Catal. 2018;13(3):560–572. doi:10.9767/bcrec.13.3.1980.560-572
DOI: https://doi.org/10.15826/chimtech.2023.10.3.01
Copyright (c) 2023 Dina N. Akbayeva, Indira A. Smagulova, Azhar Timurkyzy, Botagoz S. Bakirova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







