Copolymer of chitosan with acrylamide: electron beam stimulated synthesis, structure and properties
Abstract
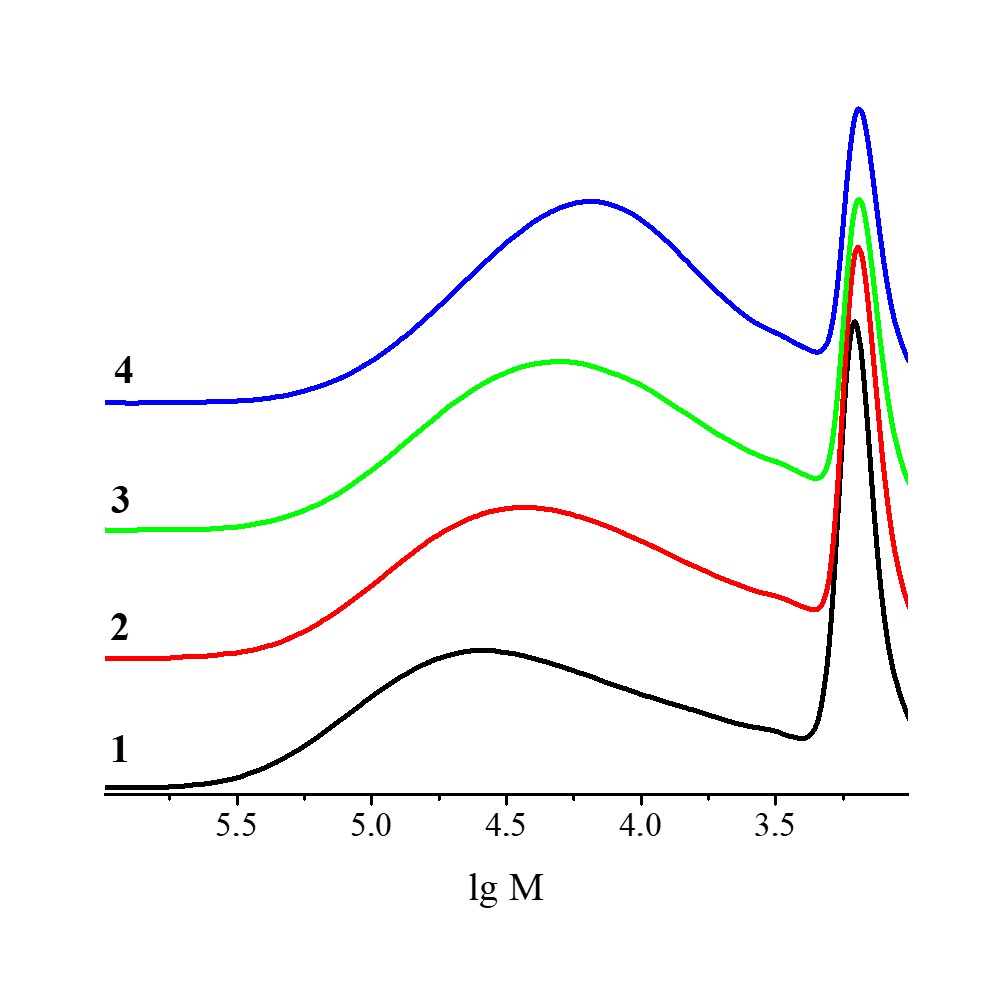
The aim of this research was to obtain the grafted copolymer of chitosan with acrylamide using the electron beam irradiation. Radiation dose was varied from 6 to 160 kGy. The highest yield of the product was observed at radiation dose of 12–40 kGy. Further increase in the dose caused a decrease in the product yield as well as its solubility in water. Using gel permeation chromatography, it was confirmed that unreacted chitosan remained in the product. NMR study of the water-soluble part of the product obtained under the doses of 6, 12, and 20 kGy showed that the length of the side chains of grafted acrylamide was about 2 elementary units. Investigation of chitosan solutions by means of dynamic light scattering revealed the presence of chitosan agglomerates in the solution. The possibility of obtaining dense films was demonstrated. Mechanical treatment of the copolymer in the ball mill caused an increase in the solubility of the samples obtained even at radiation doses of 80 and 160 kGy. It was determined by means of chromatographic methods that there were no products with low molecular weight in the ball-milled product, and unreacted chitosan did not undergo mechanocracking during the mechanical treatment.
Keywords
Full Text:
PDFReferences
Khajavian M, Vatanpour V, Castro-Munoz R, Boczkaj G. Chitin and derivative chitosan-based structures preparation strategies aided by deep eutectic solvents: A review. Carbohydr Polym. 2022;275:118702. doi:10.1016/j.carbpol.2021.118702
Bakshi PS, Selvakumar D, Kadirvelu K, Kumar NS. Chitosan as an environment friendly biomaterial – a review on recent modifications and applications. Int J Biol Macromol. 2020;150:1072–1083. doi:10.1016/j.ijbiomac.2019.10.113
Sivanesan I, Gopal J, Muthu M, Shin J, Mari S, Oh J. Green synthesized chitosan/chitosan nanoforms/nanocomposites for drug delivery applications. Polymers. 2021;13–14:2256. doi:10.3390/polym13142256
Pari R. Drug delivery applications of chitin and chitosan: a review. Environ Chem Lett. 2020;18(3):577–594. doi:10.1007/s10311-020-00963-5
Renbi B, Han W, authors. A chitosan solution and method of preparing the same. Patent WO2009035413A1. Publ. Date 19.03.2009 (Worldwide)
Thakhiew W, Champahom M, Devahastin S, Soponronnaritl S. Improvement of mechanical properties of chitosan-based films via physical treatment of film-forming solution. J Food Eng. 2015;158:66–72. doi:10.1016/j.jfoodeng.2015.02.027
Kumar D, Gihar S, Shrivash MK, Kumar P, Kundu PP. A review on the synthesis of graft copolymers of chitosan and their potential applications. Int J Biol Macromol. 2020;163:2097–2112. doi:10.1016/j.ijbiomac.2020.09.060
Boominathan T, Sivaramakrishna A. Recent advances in the synthesis, properties, and applications of modified chitosan derivatives: Challenges and opportunities. Top Curr Chem. 2021;379(3):19. doi:10.1007/s41061-021-00331-z
Concheiro A, Alvarez-Lorenzo C, Schneider HJ. Smart Materials for Drug Delivery: Complete Set.; Royal Society of Chemistry: Cambridge, 2013. 900 p. doi:10.1039/9781849734318
Morrow B, Payne G, Shen J. pH-Responsive self-assembly of polysaccharide through a rugged energy landscape. J Am Chem Soc. 2015;137(40):13024–13030. doi:10.1021/jacs.5b07761
Ibrahim AG, Sayed AZ, El-Wahab HA, Sayah MM. Synthesis of poly(acrylamide-graft-chitosan) hydrogel: optimization of the grafting parameters and swelling studies. Am J Pol Sci Tech. 2019;5(2):55–62. doi:10.11648/j.ajpst.20190502.13
Saber-Samandari S, Yilmaz O, Yilmaz EJ. Photoinduced graft copolymerization onto chitosan under heterogeneous conditions. J Macromol Sci A. 2012;49:591–598. doi:10.1080/10601325.2012.687970
Sautrot-Ba P, Razza N, Breloy L, Abbad Andaloussi S, Chiappone A, Sangermano M, He´lary C, Belbekhouche S, Coradin T, Versace D-L. Photoinduced chitosan–PEG hydrogels with long-term antibacterial properties. J Mater Chem B. 2019;7:6526–6538. doi:10.1080/10601325.2012.687970
Desnelli D, Eliza E, Mara A, Rachmat A. Synthesis of copolymer of chitosan with acrylamide as an adsorbent for heavy metal waste treatment. IOP Conf Ser: Mater Sci Eng. 2020;833:012064. doi:10.1088/1757-899X/833/1/012064
Mochalova AE, Zaborshchikova NV, Knyazev AA, Smirnova LA, Izvozchikova VA, Medvedeva VV, Semchikov YuD. Graft polymerization of acrylamide on chitosan: Copolymer structure and properties. Polym Sci A. 2006;48(9):918–923. doi:10.1134/S0965545X06090069
Mochalova AE, Smirnova LA. State of the art in the targeted modification of chitosan. Polym Sci B. 2018;60(2):131–161. doi:10.1134/S1560090418020045
Tatarinov PV, Mochalova AE, Belysheva IV, Smirnova LA, Bodrikov IV. Induced degradation of chitosan, conjugated with block copolymerization with acrylamide. Russ J Appl Chem. 2010;83(7):1294–1298. doi:10.1134/S1070427210070232
Okieimen FE. Preparation, characterization, and properties of cellulose-polyacrylamide graft copolymers. J Appl Polym Sci. 2003;89(4):913–923. doi:10.1002/app.12014
Shi A, Dai X, Jing Z. Tough and self-healing chitosan/poly(acrylamide-co-acrylic acid) double network hydrogels. Polym Sci A. 2020;62(3):228–239. doi:10.1134/S0965545X20030128
Sun H, Wirsén A, Albertsson A. Electron beam-induced graft polymerization of acrylic acid and immobilization of arginine−glycine−aspartic acid-containing peptide onto nanopatterned polycaprolactone. Biomacromolec. 2004;5–6:2275–2280. doi:10.1021/bm049703p
Ponomarev AV. Radiolysis as a powerful tool for polymer waste recycling. High Energy Chem. 2020;54(3):194–204. doi:10.1134/S0018143920030121
Mikhailenko MA, Shakhtshneider TP, Antonov IM, Myz SA, Kuznetsova SA, Bryazgin AA. Radiation-thermal synthesis of copolymers of chitosan with acrylamide as a means of betulin delivering. J Sib Fed Univ: Chem. 2022;15(3):420–430. doi:10.17516/1998-2836-0305
Al-Karawi AJM, Al-Qaisi ZHJ, Abdullah HI, Al-Mokaram AMA, Ajeel Al-Heetimi DT. Synthesis, characterization of acrylamide grafted chitosan and its use in removal of copper(II) ions from water. Carbohydrate Polymers. 2011;83:495–500. doi:10.1016/j.carbpol.2010.08.017
Vikhoreva GA, Pchelko OM, Gal’braikh LS, Rogovina SZ. The phase state and rheological properties of the chitosan-acetic acid-water system. Polym Sci B. 2001;43(6):1079–1084.
Blagodatskikh IV, Bezrodnykh EA, Abramchuk SS, Muranov AV, Sinitsyna OV, Khokhlov AR, Tikhonov VE. Short chain chitosan solutions: self-assembly and aggregates disruption effects. J Polym Res. 2013;20:73. doi:10.1007/s10965-013-0073-0
Mandal P, Mukherjee M, Shunmugam R. Effect of the aqueous-organic solvent mixtures upon super-aggregation of chitosan. J Polym Res. 2023;30:26. doi:10.1007/s10965-022-03404-9
Naito P, Ogawa Y, Kimura S, Iwata T, Wada M. Crystal transition from hydrated chitosan and chitosan/monocarboxylic acid complex to anhydrous chitosan investigated by X-ray diffraction. J Polym Sci B: Polym Phys. 2015;53:1065–1069. doi:10.1002/polb.23748
Mogilevskaya EL, Akopova TA, Zelenetskii AN, Ozerin AN. The Crystal Structure of Chitin and Chitosan. Polym Sci Ser A. 2006;48:116–123. doi:10.1134/S0965545X06020039
Mikhailenko MA, Shakhtshneider TP, Eltsov IV, Kozlov AS, Kuznetsova SA, Karacharov АА, Boldyrev VV. Supramolecular architecture of betulin diacetate complexes with arabinogalactan from Larix sibirica. Carbohydr Polym. 2016;138:1–7. doi:10.1016/j.carbpol.2015.11.047
Pandit A, Indurkar A, Deshpande C, Jain R, Dandekar P. A systematic review of physical techniques for chitosan degradation. Carbohydr Polym Technol Appl. 2021;2:100033. doi:10.1016/j.carpta.2021.100033
DOI: https://doi.org/10.15826/chimtech.2023.10.3.12
Copyright (c) 2023 Ilia M. Antonov, Mikhail A. Mikhailenko, Tatyana P. Shakhtshneider, Ilia V. Eltsov, Svetlana A. Kuznetsova, Maxim V. Zelikman, Alexander A. Bryazgin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







