Development of nanostructured catalysts for catalytic oxidative water purification from organic impurities, including phenolic compounds
Abstract
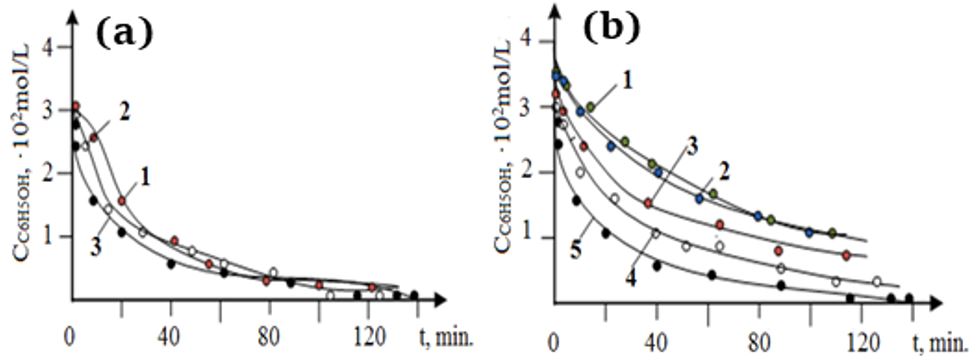
The purpose of this work was to create magnetic nanocatalysts that could be used for the oxidation of organic water pollutants – phenol and its derivatives – and to determine the physicochemical characteristics of the catalysts. The development of such active nanocomposite catalysts would solve the environmental problem in the Republic of Kazakhstan in the field of wastewater treatment from organic impurities, including phenols, and would also contribute to the subsequent creation of domestic production of oxygen-containing compounds, since almost the entire spectrum of oxygen-containing compounds for various industries is imported into the Republic. Nanosized magnetic composites based on Fe and Co were obtained by chemical deposition, in some cases, using polyethyleneimine and polyvinylpyrrolidone. It was shown that the interaction between nanoparticles and the polymer takes place in the case of a CoFe2O4 catalyst stabilized with polyvinylpyrrolidone or polyethyleneimine, which may indicate the efficient formation of nanocomposites. According to the IR study, for the CoFe2O4 nanocomposite stabilized with polyvinylpyrrolidone, the absorption bands at 735, 663, 649, 626 cm–1 are natural vibrations for the composite nanoparticles embedded in a polyvinylpyrrolidone matrix. The synthesized nanocomposites were tested in the oxidation of phenol with oxygen. The results demonstrate that the catalysts are promising both for the purification of industrial wastewater from phenol and for the synthesis of oxygen-containing compounds in the liquid phase under mild conditions.
Keywords
Full Text:
PDFReferences
Zhang Z, Malik MZ, Khan A, Ali N, Malik S, Bilal M. Environmental impacts of hazardous waste, and management strategies to reconcile circular economy and eco-sustainability. Sci Total Environ. 2022;807:150856. doi:10.1016/j.scitotenv.2021.150856
Prabhahar M, Gomathi K, Venkatesh R, Stalany V M, Vijayan DS, Sassykova LR, Sendilvelan S, Priya V S, Jijina GO, Selvaraj R. Isothermic and kinetic study on removal of methylene blue dye using anisomeles malabarica silver nanoparticles: an efficient adsorbent to purify dye-contaminated wastewater. Adsorp Sci Technol. 2022;2022:9878987. doi:10.1155/2022/9878987
Cosgrove WJ, Loucks DP. Water management: Current and future challenges and research directions. Water Resour Res. 2015;51(6):4823–4839. doi:10.1002/2014wr016869
Bierkens MFP. Global hydrology 2015: State, trends, and directions. Water Resour Res. 2015;51(7):4923–4947. doi:10.1002/2015wr017173
Sassykova LR. Technogenic emissions into the atmosphere: impact on the environment and neutralization by catalytic methods: Monograph. Qazaq university: Almaty;2018. 322.
Taghipour S, Hosseini SM, Ataie-Ashtiani B. Engineering nanomaterials for water and wastewater treatment: review of classifications, properties and applications. New J Chem. 2019;43(21):7902–7927. doi:10.1039/c9nj00157c
Sassykova LR, Aubakirov YA, Tashmukhambetova ZhKh. Actual ecological aspects of petrochemical manufactures. Qazaq University: Almaty; 2019. 352p.
Tian X, Zhang H, Hu C, Yan Y. Preparation of microfiber composite nitrogen doped carbon nanotube membranes and their degradation properties of phenol in the structured fixed bed. J Environ Chem Eng. 2023;11(1):109255. doi:10.1016/j.jece.2022.109255
Sassykova LR, Sendilvelan S, Bhaskar K, Zhumakanova AS, Aubakirov YA, Abildin TS, Kubekova Sh, Mataeva ZT, Zhakupova AA. Norms of emissions of harmful substances generated from vehicles in the different countries of the world. News Natl Acad Sci Repub Kazakhstan Ser Geol Tech Sci. 2019;434(2):181–190. doi:10.32014/2019.2518-170X.53
Baabu PRS, Kumar HK, Gumpu MB, Babu KJ, Kulandaisamy AJ, Rayappan JBB. Iron oxide nanoparticles: a review on the province of its compounds, properties and biological applications. Mater. 2023;16:59. doi:10.3390/ma16010059
Wanna WH, Janmanchi D, Thiyagarajan N, Ramu R, Tsai YF, Yu SSF. Selective oxidation of simple aromatics catalyzed by nanobiomimetic metal oxide catalysts: a mini review. Front Chem. 2020;8. doi:10.3389/fchem.2020.589178
Arefieva OD, Vasilyeva MS, Kuryavy VG, Ustinov AY, Zemnukhova LA, Gushchina DD. Oxidative destruction of phenol on Fe/SiO2 catalysts. Water Sci Technol Water Supply. 2020;81:2189–2201. doi:10.2166/wst.2020.277
Abid MF, Alwan GM, Abdul-Ridha LA. Study on catalytic wet air oxidation process for phenol degradation in synthetic wastewater using trickle bed reactor. Arab J Sci Eng. 2016;41(7):2659–2670. doi:10.1007/s13369-016-2171-x
Ayati A, Ahmadpour A, Bamoharram FF, Tanhaei B, Mänttäri M, Sillanpää M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere. 2014;107:163–174. doi:10.1016/j.chemosphere.2014.01.040
Tashmukhambetova ZK, Sassykova LR, Aubakirov YA, Dangaliyeva AK, Kanatbayeva MA, Rustem AE. New catalysts for toluene oxidation technology in the liquid phase. Mater Today Proc. 2020;31:529–531. doi:10.1016/j.matpr.2020.06.141
Dossumova BT, Shakiyeva TV, Muktaly D, Sassykova LR, Baizhomartov BB, Subramanian S. Synthesis, Characterization of magnetic composites and testing of their activity in liquid-phase oxidation of phenol with oxygen. Chem Eng. 2022;6(5):68. doi:10.3390/chemengineering6050068
Iorio ED, Colombo C, Cheng Z, Capitani G, Mele D, Ventruti G, Angelico R. Characterization of magnetite nanoparticles synthetized from Fe(II)/nitrate solutions for arsenic removal from water. J Environ Chem Eng. 2019;7(2):102986. doi:10.1016/j.jece.2019.102986
Khabibullin VR, Stepanov GV. Effect of a low-frequency magnetic field on the release of heat by magnetic nanoparticles of different shapes. Russ J Phys Chem. 2020;94(2):439–444. doi:10.1134/s0036024420020168
Sassykova LR, Sassykova AR, Kubekova ShN, Batyrbayeva AA, Azhigulova RN, Zhaxibayeva ZhM, Kozhaisakova MA, Zhusupova L A, Sendilvelan S, Ponomarenko OI. Hydrogenation of aromatic nitro compounds to amines on nickel and iron-containing catalysts. Rasayan J Chem. 2021;14(2):1223–1229. doi:10.31788/RJC.2021.1426124
Huth S, Lausier J, Gersting SW, Rudolph C, Plank C, Welsch U, Rosenecker J. Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. J Genet Med. 2004;6(8):923–936. doi:10.1002/jgm.577
Xenariou S, Griesenbach U, Ferrari S, Dean P, Scheule RK, Cheng SH, Geddes DM, Plank C, Alton EWFW. Using magnetic forces to enhance non-viral gene transfer to airway epithelium in vivo. Gene Ther. 2006;13(21):1545–1552. doi:10.1038/sj.gt.3302803
Sapir L, Stanley CB, Harries D. Properties of polyvinylpyrrolidone in a deep eutectic solvent. J Phys Chem A. 2016;120(19):3253–3259. doi:10.1021/acs.jpca.5b11927
Jung H, Kim JW, Choi H, Lee JH, Hur HG. Synthesis of nanosized biogenic magnetite and comparison of its catalytic activity in ozonation. Appl Catal B Environ. 2008;83(3–4):208–213. doi:10.1016/j.apcatb.2008.02.016
Aboelfetoh EF, El-Shenody RA, Ghobara MM. Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): reaction optimization, catalytic and antibacterial activities. Environ Monit Assess. 2017;189(7):349. doi:10.1007/s10661-017-6033-0
Catrinescu C, Teodosiu C, Macoveanu M, Miehe-Brendlé J, Dred RLe. Catalytic wet peroxide oxidation of phenol over Fe-exchanged pillared beidellite. Water Res. 2003;37(5):1154–1160. doi:10.1016/s0043-1354(02)00449-9
Zambrzycki C, Shao R, Misra A, Streb C, Herr U, Güttel R. Iron based core-shell structures as versatile materials: magnetic support and solid catalyst. Catalysts. 2021;11(1):72. doi:10.3390/catal11010072
Zielińska-Jurek A, Bielan Z, Dudziak S, Wolak I, Sobczak Z, Klimczuk T, Nowaczyk G, Hupka J. Design and application of magnetic photocatalysts for water treatment. The effect of particle charge on surface functionality. Catalysts. 2017;7(12):360. doi:10.3390/catal7120360
Stoyanova M, Christoskova St, Georgieva M. Aqueous phase catalytic oxidation of cyanides over iron-modified cobalt oxide system. Appl Catal A-Gen. 2004;274(1–2):133–138. doi:10.1016/j.apcata.2004.06.002
Naĭden EP, Zhuravlev VA, Itin VI, Terekhova OG, Magaeva AA, Ivanov YF. Magnetic properties and structural parameters of nanosized oxide ferrimagnet powders produced by mechanochemical synthesis from salt solutions. Phys Solid State. 2008;50:894–900. doi:10.1134/s1063783408050156
Svarovskaya LI, Terekhova OG, Itin VI, Magaeva AA, Naiden EP. Nanosized SnO2 and CoFe2O4 composite materials: Their preparation, properties and sorption activity. Nanotechnol Russ. 2010;5(11–12):795–799. doi:10.1134/s1995078010110078
Christoskova StG, Stoyanova M, Georgieva M. Low-temperature iron-modified cobalt oxide system. Appl Catal A-Gen. 2001;208(1–2):243–249. doi:10.1016/s0926-860x(00)00710-9
Safi R, Ghasemi A, Shoja-Razavi R. The role of shell thickness on the exchange spring mechanism of cobalt ferrite/iron cobalt magnetic nanocomposites. Ceram Int. 2017;43(1):617–624. doi:10.1016/j.ceramint.2016.09.203
Wang Y, Sun H, Ang HM, Tadé MO, Wang S. Magnetic Fe3O4/carbon sphere/cobalt composites for catalytic oxidation of phenol solutions with sulfate radicals. Chem Eng J. 2014;245:1–9. doi:10.1016/j.cej.2014.02.013
Gallo-Cordova A, Veintemillas-Verdaguer S, Tartaj P, Mazarío E, Morales MP, Ovejero J G. Engineering iron oxide nanocatalysts by a microwave-assisted polyol method for the magnetically induced degradation of organic pollutants. Nanomater. 2021;11(4):1052. doi:10.3390/nano11041052
Mardani HR, Ziari M. Synthesis and characterization of a new nanomagnetic coordination composite from Fe3O4 and Cu(II) complex: as an efficient catalyst in oxidation of benzyl alcohol. Res Chem Intermed. 2018;44:6605–6619. doi:10.1007/s11164-018-3511-0
Lin CR, Ivanova OS, Edelman IS, Knyazev YV, Zharkov SM, Petrov DA, Sokolov AE, Svetlitsky ES, Velikanov DA, Solovyov LA, Chen YZ, Tseng YT. Carbon Double Coated Fe3O4@C@C nanoparticles: morphology features, magnetic properties, dye adsorption. Nanomater. 2022;12:376. doi:10.3390/nano12030376
Geetha VT, Puthilibai G, Induja S. A starch-assisted innovative synthesis of spinel-structured and ferromagnetic behaviour of Fe3O4 nanoparticles catalytic activity evaluated in the selective oxidation. SN Appl Sci. 2019;1:472. doi:10.1007/s42452-019-0450-3
Faraji AR, Mosazadeh S, Ashouri F. Synthesis and characterization of cobalt-supported catalysts on modified magnetic nanoparticle: Green and highly efficient heterogeneous nanocatalyst for selective oxidation of ethylbenzene, cyclohexene and oximes with molecular oxygen. J Colloid Interface Sci. 2017;506:10–26. doi:10.1016/j.jcis.2017.06.100
Habibi D, Faraji AR, Arshadi M, Veisi H, Gil A. Manganese nanocatalyst and N-hydroxyphthalimide as an efficient catalytic system for selective oxidation of ethylbenzene, cyclohexene and oximes under aerobic condition. J Mol Catal A Chem. 2014;382:41–54. doi:10.1016/j.molcata.2013.10.023
Prijic S, Sersa G. Magnetic nanoparticles as targeted delivery systems in oncology. Radiol Oncol. 2011;45(1):1–16. doi:10.2478/v10019-011-0001-z
Aluker NL, Lavrentieva AL, Suzdaltseva YM. Direct optical research methods in the analytics of phenol. Opt Spectrosc. 2020;128:422–428. doi:10.1134/S0030400X20030042
Shomanova Z, Safarov R, Tashmukhambetova Z, Sassykova L, Nosenko Y, Mukanova R. Complex research of ferroalloys production wastes by physical and chemical methods. J Chem Technol Metall. 2021;56(3):629–636.
DOI: https://doi.org/10.15826/chimtech.2023.10.3.09
Copyright (c) 2023 Larissa R. Sassykova, Binara T. Dossumova, Madina S. Ilmuratova, Tatyana V. Shakiyeva, Bedelzhan B. Baizhomartov, Albina R. Sassykova, Zhanar M. Zhaxibayeva, Tleutai S. Abildin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







