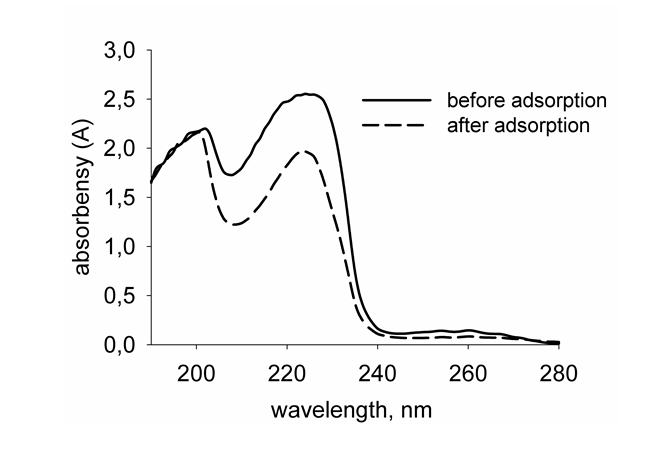
Adsorption of TX-100 and SDBS on the surface of alumina and maghemite nanoparticles from aqueous solutions
Abstract
Keywords
Full Text:
PDF (RUSSIAN) (Русский)References
Gao J, Gu H, Xu B. Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications. Acc Chem Res. 2009;42(8):1097-107. doi:10.1021/ar9000026
Zhu D, Li X, Wang N, Wang X, Gao J, Li H. Dispersion behavior and thermal conductivity characteristics of Al2O3-H2O nanofluids. Curr Appl Phys. 2009;9(1):131-9. doi:10.1016/j.cap.2007.12.008
Li X, Zhu D, Wang X. Evaluation on dispersion behavior of the aqueous copper nano-suspensions. J Colloid Interf Sci. 2007;310(2):456-63. doi:10.1016/j.jcis.2007.02.067
Mansurov RR, Leiman DV. In: Tezisy dokladov molodezhnoy nauchnoy konferentsii "Problemy teoreticheskoy i eksperimental'noy khimii" [Abstracts of XXII Russian conference for young scientists "Problems of theoretical and applied chemistry"]. 2012 May 24-28; Ekaterinburg, Russia. p. 45. Russian.
DOI: https://doi.org/10.15826/chimtech.2014.1.2.696
Copyright (c) 2014 R. R. Mansurov, A. P. Safronov, N. V. Lakiza, D. V. Leiman

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






