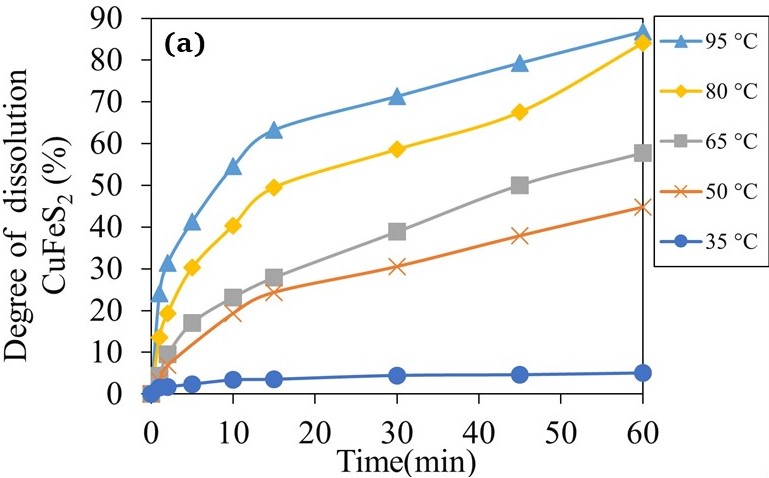
Kinetics of nitric acid leaching of bornite and chalcopyrite
Abstract
Keywords
Full Text:
PDFReferences
Japel S, Schwager B, Boehler R, Ross M. Melting of copper and nickel at high pressure: The role of d electrons. Phys Rev Lett. 2005;95(16):167801. doi:10.1103/PhysRevLett.95.167801
Khojiev S. Pyrometallurgical processing of copper slags into the metallurgical ladle. Int J Adv Res Sci. 2019;6(2):8094–8099. Available from: https://www.researchgate.net/publication/341787171
Carlesi C, Harris RC, Abbott AР, Jenkin GR. Chemical dissolution of chalcopyrite concentrate in choline chloride ethylene glycol deep eutectic solven. Minerals. 2022;12(65):1–14. doi:10.3390/min12010065
Dutrizac JE, Macdonald RJC, Ingraham TR. The kinetics of dissolution of bornite sulfate solutions. Metall Transact. 1970;1:225–231. doi:10.1007/BF02819265
Hua X, Zheng Y, Xu Q, Lu X, Cheng H, Zou X, Song Q, Ning Z. Interfacial reactions of chalcopyrite in ammonia–ammonium chloride. Transact Nonferrous Metals Soc Chi. 2018;28:556–566. doi:10.1016/S1003-6326(18)64688-6
Nguyaen K, Borja D, You J, Hong G. Chalcopyrite bioleaching using adapted mesophilic microorganisms: effects of temperature, pulp density, and initial ferrous concentrations. Mater Transact. 2018;59(11):1860–1866. doi:10.2320/matertrans.M2018247
McDonald RG, Li J, Austin PJ. High temperature pressure oxidation of a low-grade nickel sulfide concentrate with control of the residue composition. Minerals .2020;10:249. doi:10.3390/min10030249
Karimov KA, Rogozhnikov DA, Kuzas EA, Shoppert AA. Leaching kinetics of arsenic sulfide-containing materials by copper sulfate solution. Metals. 2020;18:8. doi:10.3390/met10010007
Abhilash D, Ghosh A, Pandey B. Bioleaching of low-grade granitic chalcopyrite ore by hyperthermophiles: Elucidation of kinetics-mechanism. Metall Res Technol. 2015;112(5):506. doi:10.1051/metal/2015031
Vasiliev RE, Baudouin AY, Vasilyeva AA. Hydrometallurgical technologies for processing copper raw materials. iPolytech J. 2022;4(26):677–687. doi:10.21285/1814-3520-2022-4-677-687
Johnson G, Corrans I. The Activox process for refractory gold ores. Randol Gold Forum - Beaver Creek. 1993;93:183–189.
Rogozhnikov DA, Rusalev RE, Dizer OA, Naboychenko SS. Nitric acid loosening of rebellious sulphide concentrates containing precious metals. Tsvetnye Metally. 2018;16:38–44. doi:10.17580/tsm.2018.12.05
Karimov K, Rogozhnikov D, Kuzas E, Dizer O, Golovkin D, Tretiak M. Deposition of arsenic from nitric acid leaching solutions of gold-arsenic sulphide concentrates. Metals. 2021;11:889. doi:10.3390/met11060889
Rogozhnikov DA, Karelov SV, Mamyachenkov SV, Anisimova OS. Technology for the hydrometallurgical processing of a complex multicomponent sulfide-based raw material. Metallurgist. 2013;57:247–250. doi:10.1007/s11015-013-9720-2
Agacayak T, Aras A. Leaching of chalcopyrite concentrate (CuFeS2) in nitric acid (HNO3) solution . Academ J Sci. 2013;2(1):61–65. English. Available from: https://www.researchgate.net/publication/271840766
Baba AA, Ayinla KI, Bale RB, Adekola F. Quantitative leaching of a nigerian chalcopyrite ore by nitric acid. Bayero J Pure Appl Sci. 2014;7(2):115–121. doi:10.4314/bajopas.v7i2.20
Tsogtkhangai D, Mamyachenkov SV, Anisimova OS, Naboichenko SS. Kinetics of leaching of copper concentrates by nitric acid. Russ J Non- ferrous Metals. 2012;52(6):469–472. doi:10.3103/s1067821211060162
DOI: https://doi.org/10.15826/chimtech.2023.10.4.10
Copyright (c) 2023 Yuri Shklyaev, Oleg Dizer, Tatiana Lugovitskaya, Dmitry Golovkin, Denis Rogozhnikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
© Website Chimica Techno Acta, 2014–2024
ISSN 2411-1414 (Online)
This journal is licensed under a Creative Commons Attribution 4.0 International







