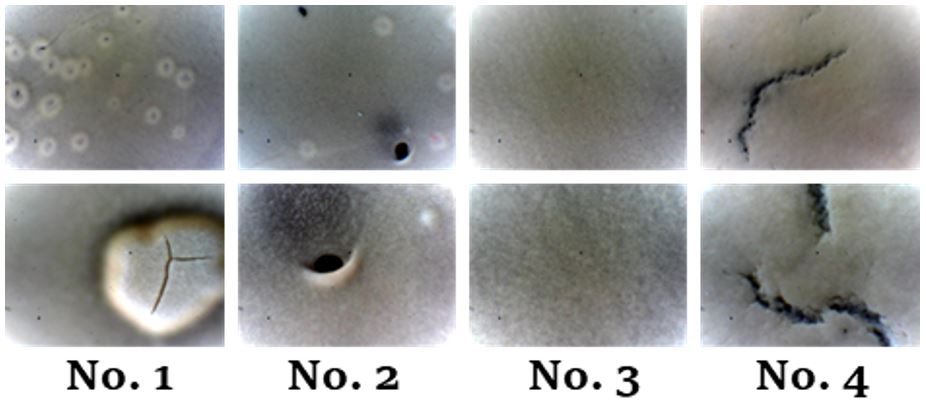
Features of forming a low-temperature cubic Li7La3Zr2O12 film by tape casting
Abstract
Keywords
Full Text:
PDFReferences
Kong L, Wang L, Zhu J, Bian J, Xia W, Zhao R, Lin H, Zhao Y. Configuring solid-state batteries to power electric vehicles: A deliberation on technology, chemistry and energy. Chem Commun. 2021;57:12587–12594. doi:10.1039/D1CC04368D
Bates AM, Preger Y, Torres-Castro L, Harrison KL, Harris SJ, Hewson J. Are solid-state batteries safer than lithium-ion batteries? Joule. 2022;6:742–55. doi:10.1016/j.joule.2022.02.007
Wu J, Yuan L, Zhang W, Li Z, Xie X, Huang Y. Reducing the thickness of solid-state electrolyte membranes for high-energy lithium batteries. Energy Environ Sci. 2021;14:12–36. doi:10.1039/D0EE02241A
Kokal I, Somer M, Notten PHL. Sol-gel synthesis and lithium ion conductivity of Li7La3Zr2O12 with garnet-related type structure. Solid State Ion. 2011;185:42–46. doi:10.1016/j.ssi.2011.01.002
Kuhn A, Narayanan S, Spencer L, Goward G. Li self-diffusion in garnet-type Li7La3Zr2O12 as probed directly by diffusion-induced 7Li spin-lattice relaxation NMR spectroscopy. Phys Rev B. 2011;83:09430201. doi:10.1103/PhysRevB.83.094302
Toda S, Ishiguro K, Shimonishi Y, Hirano A, Takeda Y, Yamamoto O, Imanishi N. Low temperature cubic garnet-type CO2-doped Li7La3Zr2O12. Solid State Ion. 2013;233:102–106. doi:10.1016/j.ssi.2012.12.007
Larraz G, Orera A, Sanjuan ML. Cubic phases of garnet type Li7La3Zr2O12: The role of hydration. J Mater Chem A. 2013;1:11419–11428. doi:10.1039/C3TA11996C
Xie H, Li Y, Goodenough JB. Low-temperature synthesis of Li7La3Zr2O12 with cubic garnet-type structure. Mater Res Bull. 2012;47:1229–1232. doi:10.1016/j.materresbull.2012.01.027
Campanella D, Belanger D, Paolella A. Beyond garnets, phosphates and phosphosulfides solid electrolytes: New ceramic perspectives for all solid lithium metal batteries. J Power Source. 2021;482:228949. doi:10.1016/j.jpowsour.2020.228949
Zheng F, Kotobuki M, Song S, Lai MO, Lu L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Source. 2018;389:198–213. doi:10.1016/j.jpowsour.2018.04.022
Yaroslavtsev AB. Solid electrolytes: Main prospects of research and development. Rus Chem Rev. 2016;85:1255–1276. doi:10.1070/RCR4634
Dunushkina LA. Introduction to methods of obtaining film electrolytes for solid oxide fuel cells: monograph. Yekaterinburg: UrB RAS; 2015. 128 p. Russian.
Tan J, Tiwari A. Fabrication and characterization of Li7La3Zr2O12 thin films for lithium ion battery. ECS Solid State Lett. 2012;1(6):57. doi:10.1149/2.013206ssl
Tadanaga K, Egawa H, Hayashi A, Tatsumisago M, Mosa J, Aparicio M, Duran A. Preparation of lithium ion conductive Al-doped Li7La3Zr2O12 thin films by a sol–gel process. J Power Sources. 2015;273:844–847. doi:10.1016/j.jpowsour.2014.09.164
Bitzer M, Gestel TV, Uhlenbruck S, Buchkremer PH. Sol-gel synthesis of thin solid Li7La3Zr2O12 electrolyte films for Li-ion batteries. Thin Solid Films. 2016;615:128–134. doi:10.1016/j.tsf.2016.07.010
Lyalin E, Il’ina E, Kalinina E, Antonov B, Pankratov A, Pereverzev D. Electrophoretic deposition and characterization of thin-film membranes Li7La3Zr2O12. Membranes. 2023;13(5):468. doi:10.3390/membranes13050468
Yi E, Wang W, Kieffer J, Richard M, Key L. Parameters governing the densification of cubic-Li7La3Zr2O12 Li+ conductors. J Power Sources. 2017;352:156–164. doi:10.1016/j.jpowsour.2017.03.126
Hanc E, Zając W, Lu L, Binggong Y, Kotobuki M, Ziąbka M, Molenda J. On fabrication procedures of Li-ion conducting garnets. J Solid State Chem. 2017;248:51–60. doi:10.1016/j.jssc.2017.01.017
Hitz GT, Dennis W. McOwen, Zhang L, Ma Z, Fu Z, Wen Y, Gong Y, Dai J, Tanner RH, Hu L, Wachsma ED. High-rate lithium cycling in a scalable trilayer Li-garnet-electrolyte architecture. Mater Today. 2019;22:50–57. doi:10.1016/j.mattod.2018.04.004
Ye R, Tsai CL, Ihrig M, Sevinc S, Rosen M, Dashjav E, Sohn YJ, Figgemeier E, Finsterbusch M. Water-based fabrication of garnet-based solid electrolyte separators for solid-state lithium batteries. Green Chem. 2020;22(15):4952–4961. doi:10.1039/d0gc01009j
Ramakumar S, Deviannapoorani C, Dhivya L, Shankar LS, Murugan R. Lithium garnets: Synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog Mater Sci. 2017;88: 325–411. doi:10.1016/j.pmatsci.2017.04.007
Meshcherskikh AN, Khaliullina ASh, Dunyushkina LA, inventors; Private Institution for the scientific development of the nuclear industry "Science and Innovation", assignee. The composition of the slurry for the production of porous ceramics. Russian Federation patent RU 2021139410. 2021 Dec 12. Russian.
Murugan R, Thangadurai V, Weppner W, Angew. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Chem Int Ed. 2007;46:7778–7781. doi:10.1002/anie.200701144
Kotobuki M, Munakata H, Kanamura K, Sato Y, Yoshida T. Compatibility of Li7La3Zr2O12 solid electrolyte to all-solid-state battery using li metal anode. J Electrochem Soc. 2010;157:1076–1079. doi:10.1149/1.3474232
Awaka J, Kijima N, Hayakawa H, Akimoto J. Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J Solid State Chem. 2009;182:2046–2052. doi:10.1016/j.jssc.2009.05.020
Il’Ina EA, Andreev OL, Antonov BD, Batalov NN. Morphology and transport properties of the solid electrolyte Li7La3Zr2O12 prepared by the solid-state and citrate–nitrate methods. J Power Sources. 2012;201:169–173. doi:10.1016/j.jpowsour.2011.10.108
Richard EM, Eric RT. Tape Casting: Theory and Practice. Wiley. 2000. 298 p.
GOST 9439-85 Polyvinyl Butyral. Technical conditions. – Moscow: Publishing House of Standards. 1985. 38 p.
Lazanas AC, Prodromidis MI. Electrochemical Impedance Spectroscopy - A Tutorial. ACS Measurement Science Au. 2023;3:162–193. doi:10.1021/acsmeasuresciau.2c00070
Shen H, Yi E, Heywood S, Parkinson DY, Chen G, Tamura N, et al. Scalable freeze-tape-casting fabrication and pore structure analysis of 3D LLZO solid-state electrolytes. ACS Appl Mater Interfaces. 2019;12:3494–3501. doi:10.1021/acsami.9b11780
Fu Z, Zhang L, Gritton JE, Godbey G, Hamann T, Gong Y, et al. Probing the mechanical properties of a doped Li7La3Zr2O12 garnet thin electrolyte for solid-state batteries. ACS Appl Mater Interfaces. 2020;12:24693–24700. doi:10.1021/acsami.0c01681
DOI: https://doi.org/10.15826/chimtech.2023.10.4.09
Copyright (c) 2023 Efim Lyalin, Larisa Pershina, Evgeniya Il’ina, Konstantin Druzhinin, Semen Belyakov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







