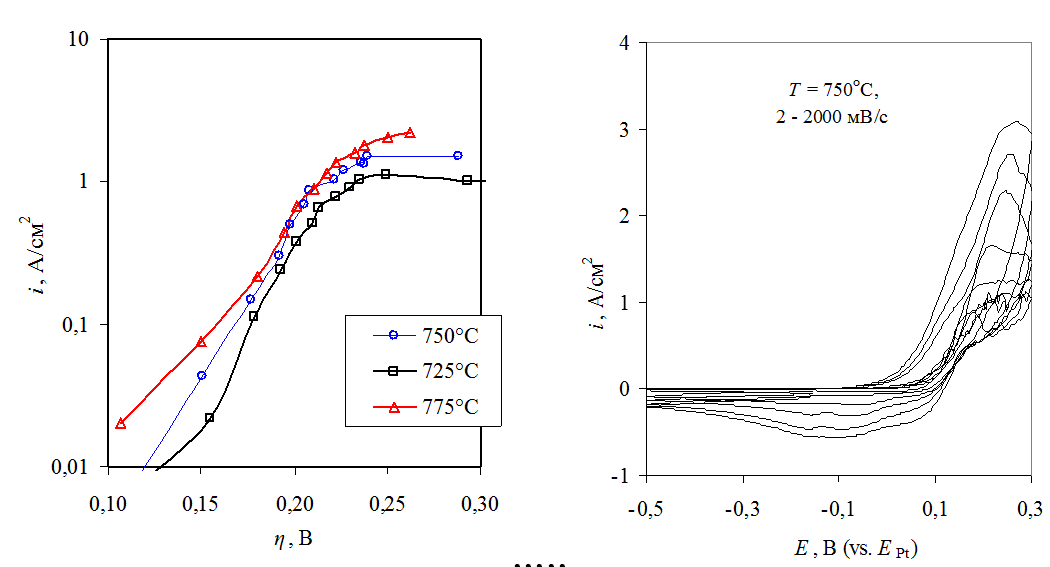
Anode process on platinum in CaCl2-CaO-based melt
Abstract
Keywords
Full Text:
PDF (RUSSIAN) (Русский)References
Chen GZ, Fray DJ. Understanding the electro-reduction of metal oxides in molten salts. In: TMS Light Metals 2004 - Proceedings of the Technical Sessions; 2004 March 14-18; United States, Carlotte, NC. p. 881-6.
Wang, D, Jin, X, Chen, GZ. Solid state reactions: An electrochemical approach in molten salts. Annu Rep Prog Chem, Sect C: Phys Chem. doi:10.1039/b703904m
Kondo H, Asaki Z, Kondo Y. Hydrolysis of fused calcium chloride at high temperature. Metall Trans B. 1978;9(4):477-83. doi:10.1007/BF02654424
Yin H, Gao L, Zhu H, Mao X, Gan F, Wang D. On the development of metallic inert anode for molten CaCl2-CaO System. Electrochim Acta. 2011;56:3296-302. doi:10.1016/j.electacta.2011.01.026
Jiao Sh, Fray DJ. Development of an inert anode for electrowinning in calcium chloride-calcium oxide melts. Metall Mater Trans B. 2010;41(1):74-9. doi:10.1007/s11663-009-9281-8
Dubtsev AB, Zaikov YuP, Batukhina VP, Ivanovsky LE. [Behaviour of the oxide anodes at the electrolysis chloride-oxide melts. 1. Interaction oxide electrodes with melt]. Rasplavy [Melts]. 1992;1:35-40. Russian.
Glushko VP. Termodinamicheskiye konstanty individual'nykh veshchestv: spravochnik [Thermodynamic constants of individual substances: a Handbook]. Moscow: Nauka; 1978-1982. 4 vol. Russian.
Rusinov LP, Gulyanitsky BS. Ravnovesnyye prevrashcheniya metallurgicheskikh reaktsiy [Equilibrium transformations of metallurgical reactions]. Moscow: Metallurgy; 1975. 416 p. Russian.
Vatolin NA, Sotnikov AI, Vatolina ND. Okislitel'no-vosstanovitel'nyye protsessy s uchastiyem ionov zheleza i kisloroda na granitse metalla s oksidnym rasplavom [Redox processes with participation of iron ions and oxygen on the boundary of metal-oxide melt]. Ekaterinburg: UrO RAN, USTU-UPI; 2008. 232 p. Russian.
Dewing EW, van der Kouwe ETh. Anodic phenomena in cryolite-alumina melts. J Electrochem Soc. 1977;124(1):58-64. doi:10.1149/1.2133245
Seriani N, Pompe W, Ciacchi LC. Catalytic oxidation activity of Pt3O4 surfaces and thin films. J Phys Chem B. 2006;110(30):14860-9. doi:10.1021/jp063281r
Scholz F. Electroanalytical Methods. Berlin: Springer-Verlag; 2010. 359 p. doi:10.1007/978-3-642-02915-8
Bard AJ, Faulkner LR. Electochemical Methods: Fundamentals and Applications. 2nd ed. New York: John Wiley and Sons Inc; 2001. 864 p.
DOI: https://doi.org/10.15826/chimtech.2014.1.3.717
Copyright (c) 2014 K. V. Tatarenko, A. V. Suzdaltsev, A. P. Khramov, Yu. P. Zaikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






