Comparative characteristic of Bi- and La- doped (Ca/Sr)MoO4 -based materials with a defect scheelite-type structure
Abstract
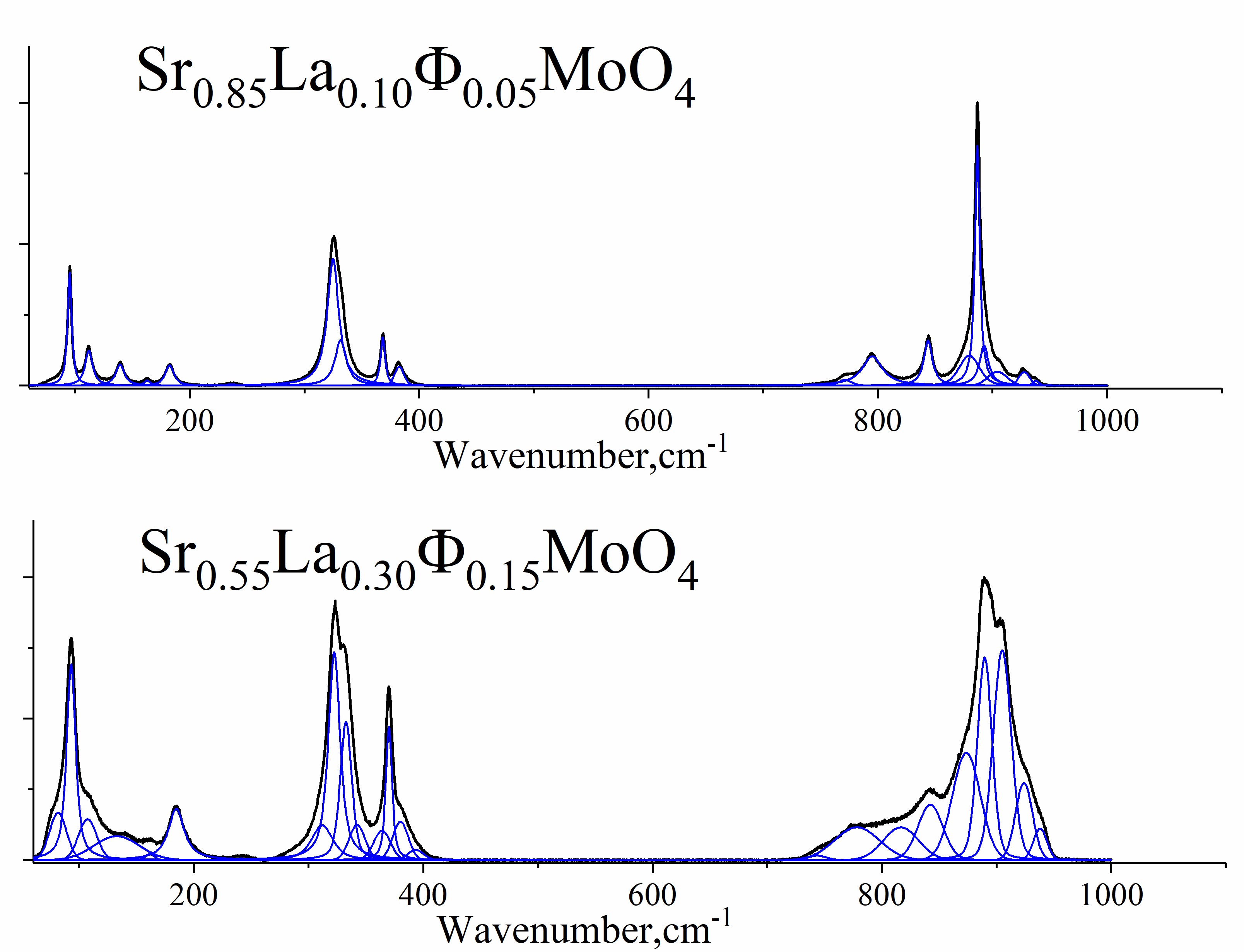
CaMoO4- and SrMoO4-based scheelite-type phases are noteworthy functional materials, whose properties strongly correlate with their structure. This work is devoted to La- or Bi-doped scheelite-type molybdates. The purpose of the present study is to quantify the effect of isolated electron pairs of bismuth on the distortion of the structure and related properties. Conventional solid-state technology was used for the synthesis of (Ca/Sr)1–3xLa2xФxMoO4 and Sr1–3xBi2xФxMoO4, (0.025≤ x ≤ 0.275). The structure was investigated by X-ray powder diffraction and Raman spectroscopy. Rates of structure distortion were characterised by the analysis of the autocorrelation function (AAF) of Raman spectra. Energy gaps were calculated by the Kubelka-Munk method. The conductivity was studied with a.c. impedance spectroscopy. For (Ca/Sr)1−3x(Bi/La)2xФxMoO4 series 0.025 ≤ x ≤ 0.15 compositions show a basic defect scheelite structure, while 0.15 < x ≤ 0.225 compositions of Bi-doped samples exhibit tetragonal supercells. The chemical compression of unit cell is more evident in the case of Bi-doping, indicating the preferred orientation of the isolated electron pairs. The distortion of MoO4 polyhedra showed by AAF was more significant for Sr1−3xBi2xФxMoO4 than for Sr1−3xLa2xФxMoO4, the Δcorr parameters for Bi-doped compositions were almost double in comparison with La-doped one in the range of 50–600 cm–1 of the Raman shift. The «critical» x = 0.15 point was also clearly indicated by Δcorr parameter. The AAF of the Raman spectra of solid oxides was shown to be a good tool for prediction of properties and points of phase transitions in solid oxides.
Keywords
Full Text:
PDFReferences
Frank M, Smetanin SN, Jelinek M, Vyhlidal D, Kopalkin AA, Shukshin VE, Ivleva LI, Zverev PG, Kubecek V. Synchronously-pumped all-solid-state SrMoO4 Raman laser generating at combined vibrational Raman modes with 26-fold pulse shortening down to 1.4 ps at 1220 nm. Opt Laser Technol. 2019;111:129–133. doi:10.1016/j.optlastec.2018.09.045
Künzel R, Umisedo NK, Okuno E, Yoshimura EM, de Azevedo Marques AP. Effects of microwave-assisted hydrothermal treatment and beta particles irradiation on the thermoluminescence and optically stimulated luminescence of SrMoO4 powders. Ceram Int. 2020;46:15018–15026. doi:10.1016/j.ceramint.2020.03.032
Yu H, Shi X, Huang L, Kang X, Pan D. Solution-deposited and low temperature-annealed Eu3+/Tb3+-doped CaMoO4/SrMoO4 luminescent thin films. Lumin J. 2020;225:117371. doi:10.1016/j.jlumin.2020.117371
Elakkiya V, Sumathi S. Low-temperature synthesis of environment-friendly cool yellow pigment: Ce substituted SrMoO4. Mater Lett. 2020;263:127246. doi:10.1016/j.matlet.2019.127246
Mikhailik VB, Elyashevskyi Yu, Kraus H, Kim HJ, Kapustianyk V, Panasyuk M. Temperature dependence of scintillation properties of SrMoO4. Nucl Instrum Methods Phy Res A. 2015;79:1–5. doi:10.1016/j.nima.2015.04.018
Danevich FA. Radiopure tungstate and molybdate crystal scintillators for double beta decay experiments. Int J Mod Phys A. 2017;32(30):1743008. doi:10.1142/S0217751X17430084
Guo HH, Zhou D, Pang LX, Qi ZM. Microwave dielectric properties of low firing temperature stable scheelite structured (Ca,Bi)(Mo,V)O4 solid solution ceramics for LTCC applications. J Eur Ceram. 2019;39:2365–2373. doi:10.1016/j.jeurceramsoc.2019.02.010
Yu-Ling Y, Xue-Ming L, Wen-Lin F, Wu-Lin L, Chuan-Yi T. Coprecipitation synthesis and photoluminescence properties of (Ca1−x−y,Lny)MoO4: xEu3+ (Ln = Y, Gd) red phosphors. J Alloys Compd. 2010;505:239–242. doi:10.1016/j.jallcom.2010.06.037
Xie A, Yuan X, Wang F, Shi Y, Li J, Liu L, Mu Z. Synthesis and luminescent properties of Eu3+-activated molybdate-based novel red-emitting phosphors for white LEDs. J Alloys Compd. 2010;501:124–129. doi:10.1016/j.jallcom.2010.04.057
Zhu Y, Zheng G, Dai Z, Zhang L, Ma Y. Photocatalytic and luminescent properties of SrMoO4 phosphors prepared via hydrothermal method with different stirring speed. J Mater Sci Techno. 2017;33:23–29. doi:10.1016/j.jmst.2016.11.019
Guo J, Randall CA, Zhou D, Zhang G, Zhang C, Jin B, Wang H. Correlation between vibrational modes and dielectric properties in (Ca1−3xBi2xΦx)MoO4 ceramics. J Eur Ceram Soc. 2015;35:4459–2264. doi:10.1016/j.jeurceramsoc.2015.08.020
Esaka T. Ionic conduction in substituted scheelite-type oxides. Solid State Ionics. 2000;136:1–9. doi:10.1016/s0167-2738(00)00377-5
Cheng J, Liu C, Cao W, Qi M, Shao G. Synthesis and electrical properties of scheelite Ca1−xSmxMoO4+δ solid electrolyte ceramics. Mater Res Bull. 2011;46:185–189. doi:10.1016/j.materresbull.2010.11.019
Fansuri H. Catalytic partial oxidation of propylene to acrolein: the catalyst structure, reaction mechanisms and kinetics[dissertation]. Perth (Australia): Curtin University of Technology; 2005. 191 p.
Sleight JAW, Aykan K. New nonstoichiometric molybdate, tungstate, and vanadate catalysts with the scheelite-type structure. J Solid State Chem. 1975;13:231–236. doi:10.1016/0022-4596(75)90124-3
Guo J, Randall AC, Zhang G, Zhou D, Chen Y, Wang H. Synthesis, structure, and characterization of new low-firing microwave dielectric ceramics: (Ca1−3xBi2xΦx)MoO4. J Mater Chem C. 2014;2:7364–7372. doi:10.1039/c4tc00698d
Mikhaylovskaya ZA, Abrahams I, Petrova SA, Buyanova ES, Tarakina NV, Piankova DV, Morozova MV. Structural, photocatalytic and electroconductive properties of bismuth-substituted CaMoO4. J Sol State Chem. 2020;291:121627. doi:10.1016/j.jssc.2020.121627
Mikhaylovskaya ZA, Buyanova ES, Petrova SA, Klimova AV. ABO4 type scheelite phases in (Ca/Sr)MoO4 - BiVO4 - Bi2Mo3O12 systems: synthesis, structure and optical properties. Chimica Techno Acta. 2021;8:20218204. doi:10.15826/chimtech.2021.8.2.04
Liang EJ, Huo HL, Wang Z, Chao MJ, Wang JP. Rapid synthesis of A2(MoO4)3 (A = Y3+ and La3+) with a CO2 laser. Solid State Sci. 2009;11:139–43. doi:10.1016/j.solidstatesciences.2008.04.008
Porotnikova N, Khrustov A, Farlenkov A, Khodimchuk A, Partin G, Animitsa I, Kochetova N, Pavlov D., Ananyev M. Promising La2Mo2O9–La2Mo3O12 composite oxygen-ionic electrolytes: interphase phenomena. ACS Appl Mater Interfaces 2022;14:6180–6193. doi:10.1021/acsami.1c20839
Chen Z, Bu W, Zhang N, Shi J. Controlled construction of monodisperse La2(MoO4)3:Yb, Tm microarchitectures with upconversion luminescent property. J Phys Chem C. 2008;112:4378–83. doi:10.1021/jp711213r
Brazdil JF. Scheelite: a versatile structural template for selective alkene oxidation catalysts. Catal Sci Technol. 2015;5:3452–3458. doi:10.1039/c5cy00387c
Antonio MR, Teller RG, Sandstrom DR, Mehicic M, Brazdil JF. Structural characterization of bismuth molybdates by X-ray absorption spectroscopy and powder neutron diffraction profile analysis. J Phys Chem. 1988;92:2939–2944. doi:10.1021/j100321a045
Kubelka P, Munk FZ. Ein beitrag zur optik der farbanstriche. Techn Phys. 1931;12:593–601.
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A. 1976;32:751–767. doi:10.1107/S0567739476001551
Vali R. Electronic properties and phonon spectra of SrMoO4. Comp Mater Sci. 2011;50:2683–2687. doi:10.1016/j.commatsci.2011.04.018
Ramarao SD, Roopas Kiran S, Murthy VRK. Structural, lattice vibrational, optical and microwave dielectric studies on Ca1−xSrxMoO4 ceramics with scheelite structure. Mater Res Bull. 2014;56:71–79. doi:10.1016/j.materresbull.2014.04.064
Porto SPS, Scott JF. Raman Spectra of CaWO4, SrWO4, CaMoO4 and SrWO4. Phys Rev. 1976;157:716–719. doi:10.1103/PHYSREV.157.716
Hardcastle FD, Wachs IE. Molecular structure of molybdenum oxide in bismuth molybdates by Raman spectroscopy. J Phys Chem. 1975;95:10763–10772. doi:10.1021/j100179a045
Jayaraman A, Wang SY, Shieh SR, Sharma SK, Ming LC. High-pressure Raman study of SrMoO4 up to 37 GPa and pressure-induced phase transitions. J Raman Spectrosc. 1995;26:451–455. doi:10.1002/jrs.1250260609
Salje EKH, Carpenter MA, Malcherek T, Boffa Ballaran T. Autocorrelation analysis of infrared spectra from minerals. Eur J Mineral. 2000;12:503–519. doi:10.1127/0935-1221/2000/0012-0503
Pankrushina EA, Kobuzov AS, Shchapova YV, Votyakov SL. Analysis of temperature-dependent Raman spectra of minerals: Statistical approaches. J Raman Spectrosc. 2020;51:1549–1562. doi:10.1002/jrs.5825
Verma A, Sharma SK. Rare-earth doped/codoped CaMoO4 phosphors: A candidate for solar spectrum conversion. Solid State Sci. 2019;96:105945. doi:10.1016/j.solidstatesciences.2019.105945
Zhang Y, Holzwarth NAW, Williams RT. Electronic band structures of the scheelite materials CaMoO4, CaWO4, PbMoO4 and PbWO4. Phys Rev B. 1998;57:12738–12750. doi:10.1103/PhysRevB.57.12738
Mikhaylovskaya ZA, Pankrushina EA, Komleva EV, Ushakov AV, Streltsov SV, Abrahams I, Petrova SA. Effect of Bi substitution on the cationic vacancy ordering in SrMoO4-based complex oxides: structure and properties. Mater Sci Eng B. 2022;281:115741. doi:10.1016/j.mseb.2022.115741
Maji BK, Jena H, Asuvathraman R, Kutty KVG. Electrical conductivity and thermal expansion behavior of MMoO4 (M = Ca, Sr and Ba). J Alloys Compd. 2015;64:475–479. doi:10.1016/j.jallcom.2015.04.054
Irvine JTS, Sinclair DC, West AR. Electroceramics: characterization by impedance spectroscopy. Adv Mat. 1990;2:132–138. doi:10.1002/adma.19900020304
Vinke I, Diepgrond J, Boukamp B, de Vries KJ, Burggraaf AJ Bulk and electrochemical properties of BiVO4. Solid State Ionics. 1992;57:83–89. doi:10.1016/0167-2738(92)90067-y
Hartmanova M, Le MT, Jergel M, SmatkoV, Kundracik F. Structure and electrical conductivity of multicomponent metal oxides having scheelite structure. Rus J Electrochem. 2009;45:621–629. doi:10.1134/s1023193509060019
DOI: https://doi.org/10.15826/chimtech.2023.10.4.11
Copyright (c) 2023 Zoya A. Mikhaylovskaya, Alexandra V. Klimova, Sofia A. Petrova, Elizaveta A. Pankrushina, Elena S. Buyanova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







