Electrochemical copper extraction from sulphate electrolyte
E. G. Demina, A. B. Darintseva, I. B. Murashova
Abstract
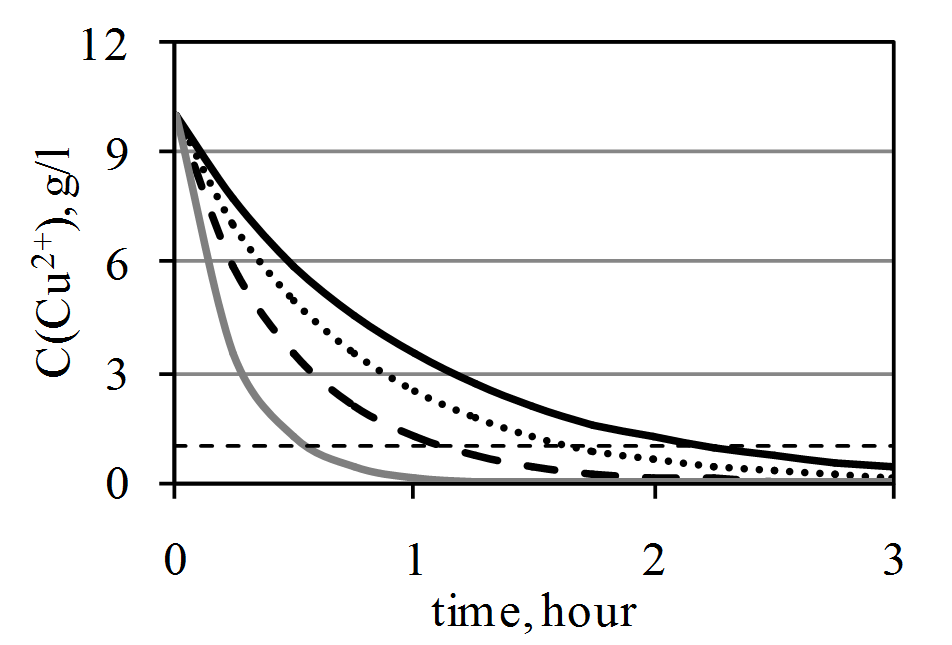
Copper and nickel ions are accumulated in the solution during electrolytic refining of copper. These ions need to be extracted from the solution from time to time. In industry copper extraction is carried out in two stages. It results in copper ions concentration reducing to 1 g/l. Numerical simulation was used to define dependence of the process of copper extraction from particular parameters: mixing of electrolyte, volume of electrolyte, area of cathodic surface. The process of copper extraction was investigated in still electrolyte and during mixing of the solution. Smooth and compact precipitate crystallized on the cathode in all experiments. Rate of copper extraction during mixing of electrolyte is higher than in still electrolyte. The industrial method of abuseive sulphate electrolyte results in the formation on the cathode dendritic sediment copper ions which are restored in extreme conditions. The decrease in the concentration of copper at I = const causes the formation of dendritic sludge that is easily crumbles to the bottom of the cell, enriching copper sludge. Guarantee smooth sediment when basmajian with obtaining compact copper, excluding besides the recovery of arsenic emitting poisonous arsina cars is the organization of intensive mixing or electrolyte circulation, ensuring the recovery of copper ions in limitless conditions smooth and sediment.
Keywords
copper refining; electrolysis; nickel; crystallization
References
Baimakov YuV, Zhurin AI. Elektroliz v gidrometallurgii [Electrolysis in hydrometallurgy]. Moscow: Metallurgizdat; 1977. 336 p. Russian.
Ogorodniychuk VI. Tsvetnye Metally. 1974;10:25. Russian.
Demina MG, Sabirov GYu, Darintseva AB, Murashova IB. In: Tezisy dokladov molodezhnoy nauchnoy konferentsii "Problemy teoreticheskoy i eksperimental'noy khimii" [Abstracts of XXIII Russian conference for young scientists "Problems of theoretical and applied chemistry"]. 2013 Apr 23-26; Ekaterinburg, Russia. p. 389. Russian.
DOI:
https://doi.org/10.15826/chimtech.2014.1.3.720
Copyright (c) 2014 E. G. Demina, A. B. Darintseva, I. B. Murashova

This work is licensed under a
Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice