Towards nanomaterials with tubular pores: synthesis and self-assembly of bis-pillar[5]arene
Abstract
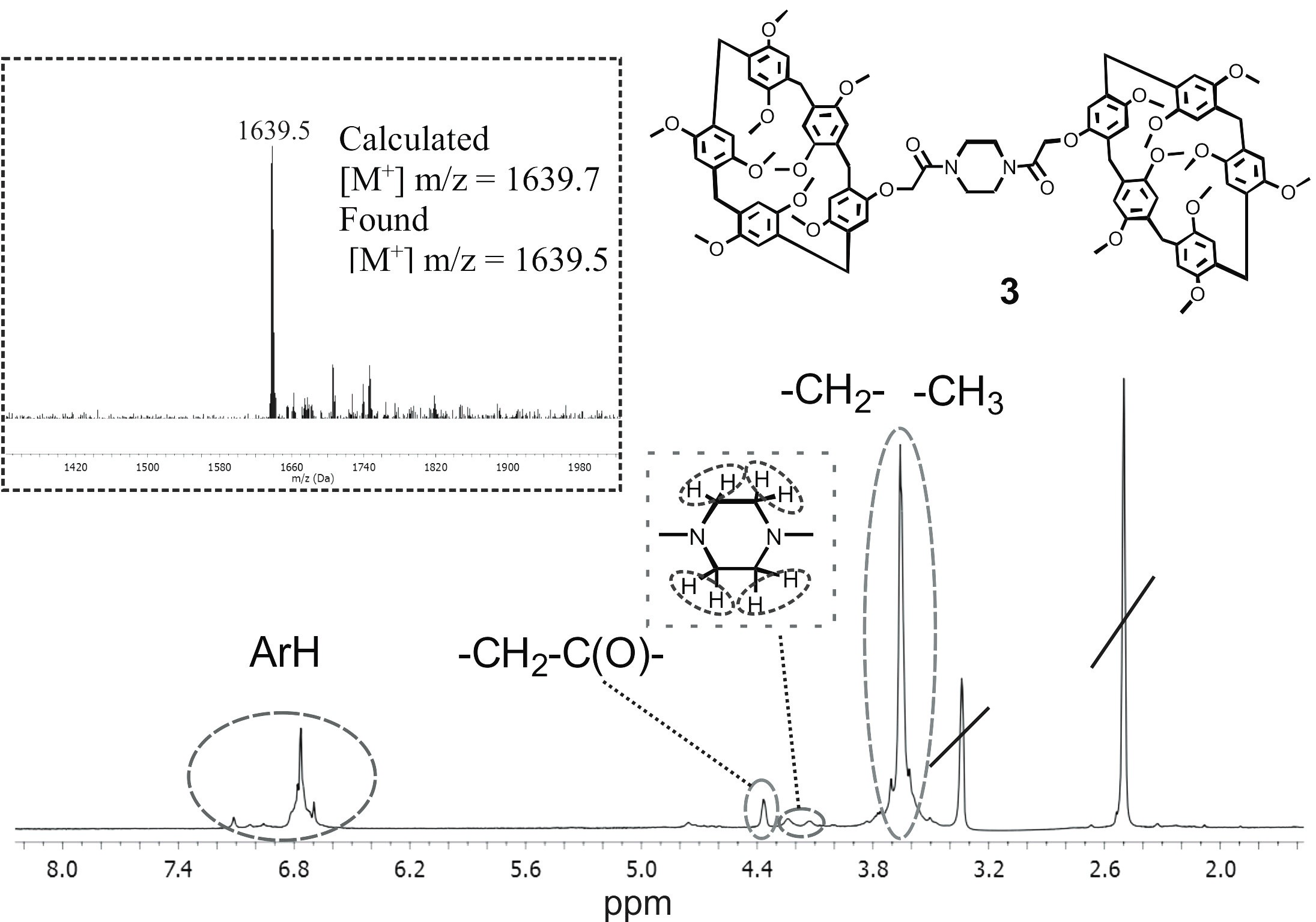
Recently, materials obtained using supramolecular chemistry approaches, and, in particular, spatially preorganized macrocyclic compounds, have attracted close attention of the researchers. Pillar[n]arenes are of special interest due to their tubular spatial structure and macrocyclic cavity. A similar tubular structure is retained in the supramolecular packaging of pillar[5]arene crystals, forming pores. In this study, we developed a block synthetic approach for the preparation of bis-pillar[5]arene containing amide groups. The ability of the synthesized bis-pillar[5]arene to form stable self-associates in solvents of different polarity (CHCl3 and CH3OH) was demonstrated by the DLS method. In trichloromethane at concentration of 1·10–3 M, monodisperse associates with average hydrodynamic diameter of 227 nm (PDI = 0.28) are formed; in methanol, stable associates (1·10–6 M) have an average hydrodynamic diameter of 136 nm (PDI = 0.21). The results obtained can be used to create new supramolecular systems, molecular machines, or capture and detect various organic molecules.
Keywords
Full Text:
PDFReferences
Hua B, Shao L, Li M, Liang H, Huang F. Macrocycle-based solid-state supramolecular polymers. Acc Chem Res. 2022;55(7):1025–1034. doi:10.1021/acs.accounts.2c00011
Olivo G, Capocasa G, Del Giudice D, Lanzalunga O, Di Stefano S. New horizons for catalysis disclosed by supramolecular chemistry. Chem Soc Rev. 2021;50(13):7681–7724. doi:10.1039/D1CS00175B
Bahl S, Nagar H, Singh I, Sehgal S. Smart materials types, properties and applications: A review. Mater Today Proc. 2020;28:1302–1306. doi:10.1016/j.matpr.2020.04.505
Clemons TD, Stupp, SI. Design of materials with supramolecular polymers. Prog Polym Sci. 2020;111:101310. doi:10.1016/j.progpolymsci.2020.101310
Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery – an underexploited structural class. Nat Rev Drug Discov. 2008;7(7):608–624. doi:10.1038/nrd2590
Ogoshi T, Kanai S, Fujinami S, Yamagishi TA, Nakamoto Y. para-Bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host–guest property. J Am Chem Soc. 2008;130(15):5022–5023. doi:10.1021/ja711260m
Shurpik DN, Aleksandrova YI, Zelenikhin PV, Subakaeva EV, Cragg PJ, Stoikov II. Towards new nanoporous biomaterials: self-assembly of sulfopillar[5]arenes with vitamin D3 into supramolecular polymers. Org Biomol Chem. 2020;18:4210–4216. doi:10.1039/D0OB00411A
Shurpik DN. Sevastyanov DA, Zelenikhin PV, Padnya PL, Evtugyn VG, Osin YN, Stoikov II. Nanoparticles based on the zwitterionic pillar[5]arene and Ag+: Synthesis, self-assembly and cytotoxicity in the human lung cancer cell line A549. Beilstein J Nanotechnol. 2020;11(1):421–431. doi:10.3762/bjnano.11.33
Shurpik DN, Makhmutova LI, Usachev KS, Islamov DR, Mostovaya OA, Nazarova AA, Kizhnyaev VN, Stoikov II. Towards universal stimuli-responsive drug delivery systems: pillar[5]arenes synthesis and self-assembly into nanocontainers with tetrazole polymers. Nanomater. 2021;11(4):947. doi:10.3390/nano11040947
Cao D, Meier H. Pillar[n]arenes – a novel, highly promising class of macrocyclic host molecules. Asian J Org Chem. 2014;3(3):244-262. doi:10.1002/ajoc.201300224
Wang M, Zhou J, Li E, Zhou Y, Li Q, Huang F. Separation of monochlorotoluene isomers by nonporous adaptive crystals of perethylated pillar[5]arene and pillar[6]arene. J Am Chem Soc. 2019;141(43):17102–17106. doi:10.1021/jacs.9b09988
Jie K, Zhou Y, Li E, Zhao R, Liu M, Huang F. Linear positional isomer sorting in nonporous adaptive crystals of a pillar[5]arene. J Am Chem Soc. 2018;140(9):3190–3193. doi:10.1021/jacs.7b13156
Tan LL, Zhang Y, Li B, Wang K, Zhang SXA, Tao Y, Yang YW. Selective recognition of “solvent” molecules in solution and the solid state by 1, 4-dimethoxypillar [5] arene driven by attractive forces. New J Chem. 2014;38(2):845–851. doi:10.1039/C3NJ01498C
Chen Y, Cao D, Wang L, He M, Zhou L, Schollmeyer D, Meier H. Monoester copillar[5]arenes: synthesis, unusual self-inclusion behavior, and molecular recognition. Chem Eur J. 2013;19(22):7064–7070. doi:10.1002/chem.201204628
Wang ZQ, Wang X, Yang YW. Pillararene‐based supramolecular polymers for adsorption and separation. Adv Mater. 2023;2301721. doi:10.1002/adma.202301721
Yang L, Tan X, Wang Z, Zhang X. Supramolecular polymers: historical development, preparation, characterization, and functions. Chem Rev. 2015;115(15):7196–7239. doi:10.1021/cr500633b
Sun M, Song P. Alicyclic tertiary amine based hyperbranched polymers with excitation-independent emission: structure, fluorescence and applications. Polym Chem. 2019;10(17):2170–2175. doi:10.1039/C9PY00183B
Zhang H, Liu Z, Fu H. Pillararenes trimer for self-assembly. Nanomater. 2020;10(4):651. doi:10.3390/nano10040651
Ravdel AA, Ponomarev AM. Brief reference book of physical and chemical quantities. Tenth edition, rev. and additional. “Ivan Fedorov”: St. Petersburg; 2003. P. 159. Russian.
Ogoshi T, Kakuta T, Yamagishi TA. Applications of pillar[n]arene‐based supramolecular assemblies. Angew Chem Int Ed. 2019;58(8):2197–2206. doi:10.1002/anie.201805884
Yakimova LS, Shurpik DN, Guralnik EG, Evtugyn VG, Osin YN, Stoikov II. Fluorescein‐loaded solid lipid nanoparticles based on monoamine pillar[5]arene: synthesis and interaction with DNA. ChemNanoMat. 2018;4(9):919–923. doi:10.1002/cnma.201800207
Aleksandrova YI, Shurpik DN, Mostovaya OA, Nazmutdinova VA, Vakhitov IR, Gerasimov AV, Islamov DR, Kuzin YI, Evtugyn GA, Stoikov II. Metallo‐supramolecular structures binding CO2: self‐assembly of fluorescent nanoparticles based on pyridine derivatives of pillar[5]arene and Cu (I). ChemNanoMat. 2023;9(7):e202300124. doi:10.1002/cnma.202300124
DOI: https://doi.org/10.15826/chimtech.2023.10.4.12
Copyright (c) 2023 Dmitriy N. Shurpik, Yulia I. Aleksandrova, Lyaysan I. Makhmutova, Alan A. Akhmedov, Ivan I. Stoikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
© Website Chimica Techno Acta, 2014–2024
ISSN 2411-1414 (Online)
This journal is licensed under a Creative Commons Attribution 4.0 International







