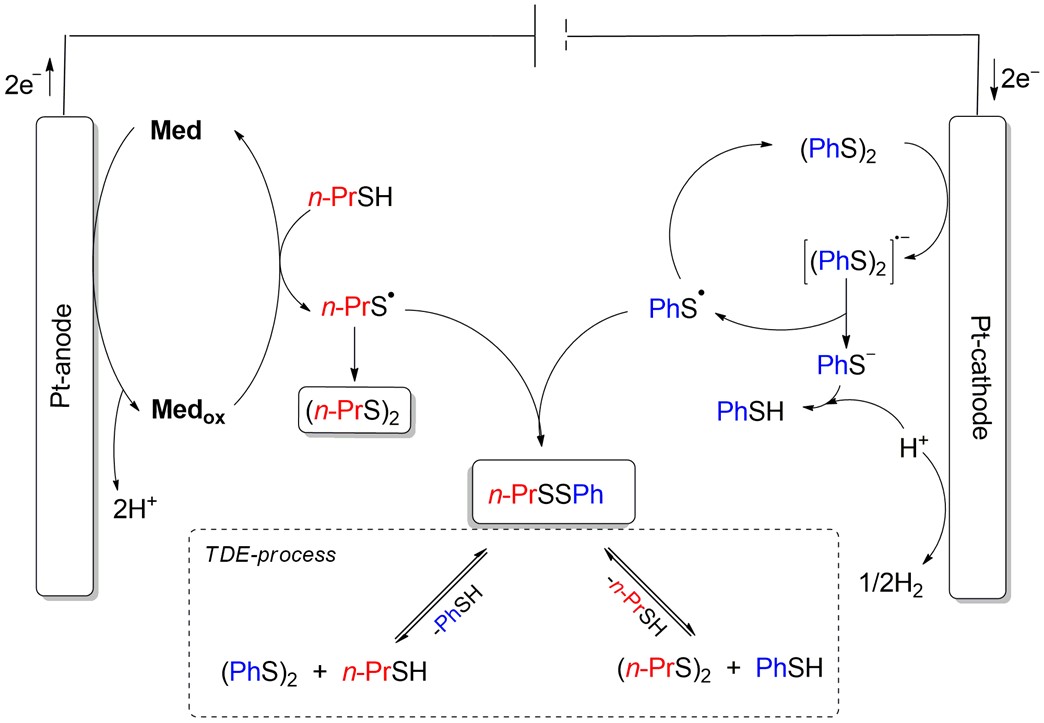
The transformations of thiols and their dimers in the redox-mediated thiol-disulfide exchange reaction
Abstract
Keywords
Full Text:
PDFReferences
Feng M, Tang B, Liang SH and Jiang X. Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr Top Med Chem. 2016;16(11):1200–1216. doi:10.2174/1568026615666150915111741
Mustafa M, Winum JY. The importance of sulfur-containing motifs in drug design and discovery. Expert Opin Drug Discov. 2022;17(5):501–512. doi:10.1080/17460441.2022.2044783
Deng L, Li X, Miao K, Mao X, Han M, Li D, Mu C, Ge L. Development of disulfide bond crosslinked gelatin/ε-polylysine active edible film with antibacterial and antioxidant activities. Food Bioprocess Technol. 2020;13:577–588. doi:10.1007/s11947-020-02420-1
Miękus N, Marszałek K, Podlacha M, Iqbal A, Puchalski C, Świergiel AH. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molec. 2020;25:3804. doi:10.3390/molecules25173804
Nghiem TT, Nguyen BL, Huyen LT, Kawahara S. A novel approach to prepare self-healing vulcanized natural rubber using tetramethylthiuram disulfide. Polym J. 2023;55:1097–1102. doi:10.1038/s41428-023-00818-0
Dong R, Pfeffermann M, Skidin D, Wang F, Fu Y, Narita A, Tommasini M, Moresco F, Cuniberti G, Berger R, Müllen K, Feng X. Persulfurated coronene: a new generation of “sulflower”. J Am Chem Soc. 2017;139:2168−2171. doi:10.1021/jacs.6b12630
Patil NA, Tailhades J, Hughes RA, Separovic F, Wade JD, Hossain MA. Cellular disulfide bond formation in bioactive peptides and proteins. Int J Mol Sci. 2015;16:1791–1805. doi:10.3390/ijms16011791
Góngora-Benítez M, Tulla-Puche J, Albericio F. Multifaceted roles of disulfide bonds. Peptides as therapeutics. Chem Rev. 2014;114:901−926. doi:10.1021/cr400031z
Mandal B, Basu B. Recent advances in S–S bond formation. RSC Adv. 2014;4:13854–13881. doi:10.1039/C3RA45997G
Wang M, Jiang X. Sulfur–sulfur bond construction. Top Curr Chem (Z). 2018;376:14. doi:10.1007/s41061-018-0192-5
Ong CL, Titinchi S, Juan JC, Khaligh NG. An Overview of recent advances in the synthesis of organic unsymmetrical disulfides. Helv Chim Acta. 2021;104(8):e2100053. doi:10.1002/hlca.202100053
Harusawa S, Yoshida K, Kojima C, Araki L, Kurihara T. Design and synthesis of an aminobenzo-15-crown-5-labeled estradiol tethered with disulfide linkage. Tetrahedron. 2004;60:11911–11922. doi:10.1016/j.tet.2004.09.109
Mu YQ, Nodwell M, Pace JL, Shaw JP, Judice JK. Vancomycin disulfide derivatives as antibacterial agents. Bioorg Med Chem Lett. 2004;14:735–738. doi:10.1016/j.bmcl.2003.11.040
Morais GR, Falconer RA. Efficient one-pot synthesis of glycosyl disulfides. Tetrahedron Lett. 2007;48:7637–7641. doi:10.1016/j.tetlet.2007.08.106
Yang F, Wang W, Li K, Zhao W, Dong X. Efficient one-pot construction of unsymmetrical disulfide bonds with TCCA. Tetrahedron. 2017;58:218–222. doi:10.1016/j.tetlet.2016.12.007
Yuan J, Liu C, Lei A. Oxidative cross S–H/S–H coupling: selective synthesis of unsymmetrical aryl tert-alkyl disulfanes. Org Chem Front. 2015;2:677–680. doi:10.1039/C5QO00027K
Vandavasi JK, Hu WP, Chen CY, Wang JJ. Efficient synthesis of unsymmetrical disulfides. Tetrahedron. 2011;67:8895–8901. doi:10.1016/j.tet.2011.09.071
Burmistrova DA, Smolyaninov IV, Berberova NT. Directed oxidative coupling of thiols in the synthesis of unsymmetrical disulfides. Russ Chem Bull. 2020;69:990–995. doi:10.1007/s11172-020-2860-1
Wang D, Liang X, Xiong M, Zhu H, Zhou Y, Pan Y. Synthesis of unsymmetrical disulfides via PPh 3-mediated reductive coupling of thiophenols with sulfonyl chlorides. Org Biomol Chem. 2020;18:4447–4451. doi:10.1039/D0OB00804D
Xu Y, Shi X, Wu L. tBuOK-triggered bond formation reactions. RSC Adv. 2019;9:24025–24029. doi:10.1039/C9RA04242C
Qiu X, Yang X, Zhang Y, Song S, Jiao N. Efficient and practical synthesis of unsymmetrical disulfides via base-catalyzed aerobic oxidative dehydrogenative coupling of thiols. Org Chem Front. 2019;6:2220–2225. doi:10.1039/C9QO00239A
Mayer CD, Allmendinger L, Bracher F. Synthesis of novel steroid analogues containing nitrile and disulfide moieties via palladium-catalyzed cross-coupling reactions. Tetrahedron. 2012;68:1810–1818. doi:10.1016/j.tet.2011.11.076
Liu C, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, Xian M. Capture and Visualization of Hydrogen Sulfide by a Fluorescent Probe. Angew Chem. 2011;123;10511–10513. doi:10.1002/ange.201104305
Yue H, Wang J, Xie Z, Tian J, Sang D, Liu S. 1,3‐Diisopropylcarbodiimide‐mediated synthesis of disulfides from thiols. ChemistrySelect. 2020;5:4273–4277. doi:10.1002/slct.202000638
Musiejuk M, Witt D. Recent developments in the synthesis of unsymmetrical disulfanes (disulfides). A review. Org Prep Proced Int. 2015;47(2):95–131. doi:10.1080/00304948.2015.1005981
Guo J, Zha J, Zhang T, Ding CH, Tan Q, Xu B. PdCl2/DMSO-catalyzed thiol–disulfide exchange: synthesis of unsymmetrical disulfide. Org Lett. 2021;23:3167–3172. doi:10.1021/acs.orglett.1c00858
Tanaka K, Ajiki K. Phosphine-free cationic rhodium(I) complex-catalyzed disulfide exchange reaction: convenient synthesis of unsymmetrical disulfides. Tetrahedron Lett. 2004;45:5677–5679. doi:10.1016/j.tetlet.2004.05.092
Itoh T, Tsutsumi N, Ohsawa A. Disproportionation reaction of disulfides promoted by nitric oxide (NO) in the presence of oxygen. Bioorg Med Chem Lett. 1999;9(15):2161–2166. doi:10.1016/S0960-894X(99)00350-9
Huang P, Wang P, Tang S, Fu Z., Lei A. Electro-oxidative S−H/S−H cross-coupling with hydrogen evolution: facile access to unsymmetrical disulfides. Angew Chem. 2018;130(27):8247–8251. doi:10.1002/ange.201803464
Wang Y, Deng L, Mei H, Bu B, Han J, Pan Y. Electrochemical oxidative radical oxysulfuration of styrene derivatives with thiols and nucleophilic oxygen sources. Green Chem. 2018;20:3444–3449. doi:10.1039/C8GC01337C
Sidiq N, Bhat MA, Khan KZ, Khuroo MA. Microwave‐assisted synthesis of disulfides using tetrathiomolybdate: A step toward greener synthesis. Heteroatom Chem. 2012;23:373–376. doi:10.1002/hc.21025
Dethe DH, Srivastava A, Dherange BD, Kumar BV. Unsymmetrical disulfide synthesis through photoredox catalysis. Adv Synth Catal. 2018;360:3020–3025. doi:10.1002/adsc.201800405
Spiliopoulou N, Kokotos CG. Photochemical metal-free aerobic oxidation of thiols to disulfides. Green Chem. 2021;23:546–551. doi:10.1039/D0GC03818K
Shatskiy A, Lundberg H, Kärkäs MD. Organic electrosynthesis: applications in complex molecule synthesis. ChemElectroChem. 2019;6(16):4067–4092. doi:10.1002/celc.201900435
Leech MC, Garcia AD, Petti A, Dobbs AP, Lam K. Organic electrosynthesis: from academia to industry. React Chem Eng. 2020;5:977–990. doi:10.1039/D0RE00064G
Amri N, Wirth T. Recent advances in the electrochemical synthesis of organosulfur compounds. Chem Rec. 2021;21:2526–2537. doi:10.1002/tcr.202100064
He M, Zhong P, Liu H, Ou C, Pan Y, Tang H. Electrochemically mediated three-component synthesis of isothioureas using thiols as sulfur source. Green Synth Cat. 2023;4(1):41–45. doi:10.1016/j.gresc.2022.03.002
Zhang YZ, Mo ZY, Wang HS, Wen XA, Tang HT, Pan YM. Electrochemically enabled chemoselective sulfonylation and hydrazination of indoles. Green Chem. 2019;21:3807–3811. doi:10.1039/C9GC01201J
Mo ZY, Zhang YZ, Huang GB, Wang XY, Pan YM, Tang HT. Electrochemical Sulfonylation of Alkynes with Sulfonyl Hydrazides: A Metal- and Oxidant-Free Protocol for the Synthesis of Alkynyl Sulfones. Adv Synth Catal. 2020;362:2160–2167. doi:10.1002/adsc.201901607
Do QT, Elothmani D, Le Guillanton G, Simonet J. A new electrochemical method of preparation of unsymmetrical disulfides. Tetrahedron Lett. 1997;38:3383–3384. doi:10.1016/S0040-4039(97)00624-2
Burmistrova DA, Smolyaninov IV, Berberova NT. Redox properties and reactivity of organic trisulfides in reactions with alkenes. Russ J Electrochem. 2020;56(4):329–336. doi:10.1134/S1023193520040035
Li Y, Wang H, Wang Z, Alhumade H, Huang Z. Electrochemical radical-mediated selective C(sp3)–S bond activation. Chem Sci. 2023;14:372–378. doi:10.1039/D2SC05507D
Lavrent’ev VA, Shinkar’ EV, Smolyaninov IV, Ryabukhin YuI, Berberova NT. Antimony(V) and Tin(IV) complexes with redox-active O,N,O-donor ligand in the electrosynthesis of symmetrical disulfides. Russ J Coord Chem. 2021;47:341–346. doi:10.1134/S1070328421050031
Sun XJ, Yang SF, Wang ZT, Liang S, Tian HY, Yang SX, Liu YG, Sun BG, Zeng CC. Electrochemically oxidative coupling of S–H/S–H for S–S bond formation: a facile approach to diacid-disulfides. ChemistrySelect. 2020;5:4637–4641. doi:10.1002/slct.202000872
Burmistrova DA, Galustyan A, Smolyaninov IV, Berberova N.T. Substituted o-Aminophenols as redox-mediators in the thiol oxidation to unsymmetrical disulfides. J Electrochem Soc. 2021;168(5):055501. doi:10.1149/1945-7111/abfe43
Burmistrova DA, Galustyan A, Smolyaninov IV, Berberova NT. Redox-mediated and microwave-assisted thiol activation: two approaches to unsymmetrical disulfides synthesis. J Electrochem Soc. 2022;169(11):116501. doi:10.1149/1945-7111/ac9d69
Piskunov AV, Tsys KV, Chegerev MG, Cherkasov AV. Tin(II) Complexes based on N-alkyl-substituted o-amidophenolate ligands: acid–base and redox transformations. Russ J Coord Chem. 2019;45:626–636. doi:10.1134/S1070328419090069
Abakumov GA, Chegerev MG, Piskunov AV, Starikova AA. Kubrin SP, Fukin GK, Cherkasov VK, Abakumov GA. Redox isomerism in main‐group chemistry: tin complex with o‐iminoquinone ligands. Eur J Inorg Chem. 2018;9:1087–1092. doi:10.1002/ejic.201701361
Gordon AJ, Ford RA. The Chemist's Companion. Wiley Intersci. Publ., New York; 1972. 541 p.
DOI: https://doi.org/10.15826/chimtech.2023.10.4.18
Copyright (c) 2023 Daria A. Burmistrova, Ivan V. Smolyaninov, Nadezhda T. Berberova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







