A review on agrowaste based activated carbons for pollutant removal in wastewater systems
Abstract
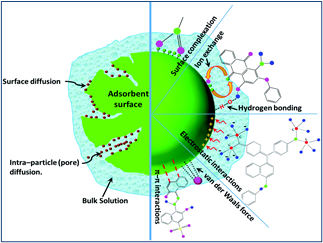
Environmental pollution from chemicals utilized in manufacturing, pharmaceuticals, and chemical process industries is of serious concern nowadays due to the contamination that ensues when these chemicals are discharged into water bodies. Activated carbon adsorption provides an efficient and economically viable means for mitigation of toxic chemicals (i.e., heavy metals, dyes, pharmaceutics, and antibiotics). However, the exorbitant cost of commercial activated carbons has resulted in the search for low-cost alternatives for the treatment of contaminated effluents. An exhaustive literature survey in this area is necessary to know the extent of work done in this area and seek out the gaps that future research will provide answers to. In this review, various works on activated carbon utilization, batch adsorption, fixed-bed adsorption (experimental and numerical studies) are summarized. This review elucidates the different kinetic and isotherm models of agrowastes-derived activated carbon materials in context with pollutants (dyes, heavy metals, pharmaceuticals, miscellaneous adsorbates) removal through batch and column methods. In addition, fixed-bed column adsorption/regeneration methods using various activated carbons derived from agrowastes are discussed. Among these methods, heavy metal adsorption from aqueous solutions by the activated carbons is the most efficient. The deployment of mathematical and machine learning approaches (ANN and novel GMDH algorithms) in optimization of batch and continuous adsorption processes are also highlighted. Numerical simulation of fixed-column adsorption systems for more improved industrial-scale column designs is described. Conclusions and future challenges of chemicals removal from polluted wastewater utilizing agrowaste-derived activated carbons are also presented.
Keywords
Full Text:
PDFReferences
McCabe WL, Smith JC, Harriott P. Unit operations of chemical engineering. 5th ed. New York: McGraw-Hill; 2005. 1130 p.
Geankoplis, C. J. Transport processes and unit operations. 3rd ed. New Jersey: PTR Prentice Hall. 1993. 921 p.
Bahadir T, Bakan G, Altas L, Buyukgungor H. The investigation of lead removal by biosorption. An application at storage battery industry wastewaters. Enzyme Microb Technol. 2007;41(1–2):98–102. doi:10.1016/j.enzmictec.2006.12.007
Nwabanne JT, Igbokwe PK. Adsorption performance of packed bed column for the removal of Lead (II) using oil palm fibre. Int J Appl Sci Technol. 2012;2(5):106–114.
Adsorbers. Visual encyclopedia of chemical engineering equipment [Internet] [cited 2024]. Available from: https://encyclopedia.che.engin.umich.edu/adsorbers, Accessed on 12 January 2024
Zulfadhly Z, Mashitah MD, Bhatia S. Heavy metals removal in fixed-bed column by the macrofungus Pycnoporus sanguineus. Env Pol. 2001;112(3):463–470. doi:10.1016/S0269-7491(00)00136-6
Kafshgari F, Keshtkar AR, Mousavian MA. Study of MO(VI) removal from aqueous solution. Application of different mathematical models to continuous adsorption data. Iran J Environ Sci Eng. 2010;10(1):1–11. doi:10.1186/1735-2746-10-14
Barros DMAS, Arroyo PA, Silva EA. (2013). General aspects of aqueous sorption process in fixed beds. In Nakajima H. Mass Transfer-Advances in sustainable energy and environment oriented numerical modeling. 1st ed. InTech. 2013. doi:10.5772/519541
Ferrarezzi CG, Guirardello R. Simulation of fixed-bed adsorption column with axial particle diameter profile for removal of solutes at low concentration. Brazil J Chem Eng. 2022;39(3):743–758. doi:10.1007/s43153-021-00168-5
Nouh SA, Lau KK, Shariff AM. Modeling and simulation of fixed bed adsorption column using in tegrated CFD approach. J Appl Sci. 2010;10(24):3229–3235. doi:10.3923/jas.2010.3229.3235
Mesfer MKA, Danish M, Khan MI, Ali IH, Hasan M, Jery AE. Continuous fixed bed CO2 adsorption: Breakthrough, column efficiency, mass transfer zone. Processes. 2020;8(10):1233–1249. doi:10.3390/pr8101233
Jani Y. Adsorption: A cost-effective wastewater treatment technology for removal of conventional and emerging organic contaminants. Ist ed. Cham: Springer International Publishing. 2022;118:17–33. doi:10.1007/698_2022_867
Ganjoo R, Sharma S, Kumar A, Daouda MMA. Activated carbon: fundamentals, classification, and properties. In Verma C, Qurashi MA. 1st eds. London: Royal Society of Chemistry; 2023. 22 p. doi:10.1039/BK9781839169861-00001
Yahya MD, Abubakar H, Obayomi KS, Iyaka YA, Suleiman B. Simultaneous and continuous biosorption of Cr and Cu(II) ions from industrial tannery effluent using almond shell in a fixed bed column. Res Eng. 2020;6:100–113. doi:10.1016/j.rineng.2020.100113
Abd AA, Othman MR, Kim J. A review on application of activated carbons for carbon dioxide capture: Present performance, preparation, and surface modification for further improvement. Env Sci Pol Res.2021;28(32):43329–43364. doi:10.1007/s11356-021-15121-9
Huang YT, Lee LC, Shih MC. A study on the pseudo-second-order kinetic equation for the adsorption of methylene blue onto nitric acid-treated rice Husk: Comparison of linear Methods. Int J of Sci and Res Pub. 2018;8(6): 7865–7877. doi:10.29322/IJSRP.8.6.2018.p7865
Liu W, Qi M, Chu X, Peng S, Han D. Investigation of adsorption-diffusion behaviors of elementary O2, CO2, and N2 in coal particles: Influence from temperature. Env Sci Pol Res.2023;30(32):78619–78631. doi:10.1007/s11356-023-7949-4
Tran HN, You SJ, Chao HP. (2016). Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J Env Chem Eng. 2016;4(3):2671–2682. doi:10.1016/j.jece.2016.05.009
Yang X, Wan Y, Zheng Y, He F, Yu Z, Huang J, Wang, H, Ok YS, Jiang Y, Gao B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem Eng J 2021;366;608–621. doi:10.1016/j.cej.2019.02.119
Dutta S, Gupta B, Srivastava SK, Gupta AK. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater Adv. 2021;2(14):4497–4531. doi:10.1039/D1MA00354B
Suzuki M. Adsorption engineering. 1st ed. Amsterdam: Elsevier. 1990. 295 p.
Slejko FL. Adsorption technology: A step-by-step approach to process evaluation and application. 1st ed. New York: M.Dekker. 1985. 223 p.
Marsh H, Rodríguez-Reinoso F. Activated carbon. 1st ed. Amsterdam: Elsevier. 2006. 536 p. doi:10.1016/B978-008044463-5/50015-7
Lima ÉC, Adebayo, MA, Machado, FM. Kinetic and equilibrium models of adsorption. In Bergmann CP, Machado FM. Carbon nanomaterials as adsorbents for environmental and biological applications. 2024:33–69. doi:10.1007/978-3-319-18875-1_3
Dotto GL, Salau NPG, Piccin JS, Cadaval TRS, de Pinto LAA. Adsorption kinetics in liquid phase: Modeling for discontinuous and continuous systems. In Bonilla- Petriciolet A, Mendoza-Castillo DI, Reynel-Ávila HE. Adsorption processes for water treatment and purification. 1st ed. Cham:nSpringer International Publishing. 2017:53–76. doi:10.1007/978-3-319-58136-1_3
Zhu Y, Kolar, P, Shah SB, Cheng JJ, Lim PK. Avocado seed-derived activated carbon for mitigation of aqueous ammonium. Ind Crops Prod. 2016;92:34–41. doi:10.1016/j.indcrop.2016.07.016
Liu G, Dai Z, Liu X, Dahlgren RA, Xu J. Modification of agricultural wastes to improve sorption capacities for pollutant removal from water-a review. Carbon Res. 2022;1(1):25–49. doi:10.1007/s44246-022-00025-1
Ruthven, DM, Kärger J, Brandani S, Mangano E. Sorption kinetics: Measurement of surface resistance. Adsorption. 2021;27(5):787–799. doi:10.1007/s10450-020-00257-w
Wang J, Guo X. Review on the intra-particle diffusion adsorption kinetics model: Interpretation, solving methods and applications. SSRN J. 2022. doi:10.2139/ssrn.4120203
Boyd G, Adamson A, Myers L. The exchange adsorption of ions from aqueous solutions by organic zeolites. II kinetics. J Am Chem Soc.1947;69:2836–2844. doi:10.1021/ja01203a066
Viegas, RMC, Campinas M, Costa H, Rosa MJ. How do the HSDM and Boyd’s model compare for estimating intraparticle diffusion coefficients in adsorption processes. Adsorpt. 2014;20(5–6):737–46. doi:10.1007/s10450-014-9617-9
Kooh MRR, Lim LBL, Lim LH, Bandara JMRS. Batch adsorption studies on the removal of malachite green from water by chemically modified Azolla pinnata. Des Wat Tr. 2015;57(31):1–13. doi:10.1080/19443994.2015.1065450
García-Mateos FJ, Ruiz-Rosas R, Marqués MD, Cotoruelo LM, Rodríguez-Mirasol J, Cordero T. Removal of paracetamol on biomass-derived activated carbon: Modeling the fixed bed breakthrough curves using batch adsorption experiments. Chem Eng J. 2015;279:18–30. doi:10.1016/j.cej.2015.04.144
Queiroz V, de Almeida DS, de Oliveira Miglioranza GH, Steffani E, Barbosa-Coutinho E, Schwaab M. Analysis of commonly used batch adsorption kinetic models derived from mass transfer-based modelling. Environ Sci Pollut Res. 2022;29(53):79875–79889. doi:10.1007/s11356-021-18479-y
Rout DR, Jena HM. Removal of phenol from aqueous solution using reduced graphene oxide as adsorbent: Isotherm, kinetic, and thermodynamic studies. Environ Sci Pol Res. 2022;29(21):32105–321119. doi:10.1007/s11356-021-17944-y
Obradovic B. Guidelines for general adsorption kinetics modeling. Hemijska Industrija [Industrial chemistry]. 2020;74(1):65–70. Russian. doi:10.2298/HEMIND200201006O
Zamri NII, Zulmajdi SLN, Daud NZA, Mahadi AH, Kusrini E, Usman A. Insight into the adsorption kinetics, mechanism, and thermodynamics of methylene blue from aqueous solution onto pectin-alginate-titania composite microparticles. SN Appl Sci. 2021;3(2):1–16. doi:10.1007/s42452-021-04245-9
Matthews AP, Weber WJJ. Effects of external mass transfer and intra-particle diffusion on adsorption rates in slurry reactors. AIChE Sym Ser. 1976;166:91–107.
Qiu Y, Zheng Z, Zhou Z, Sheng GD. Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresource Technol. 2009;100(21):5348–5351. doi:10.1016/j.biortech.2009.05.054
Kwon S. Biological pretreatment of produced water for reuse applications[dissertation]. Texas (United States of America): University of Texas at Austin; 2007. 191 p.
Cerutti JH, Parter SV. Collocation methods for parabolic partial differential equations in one space dimension. Numerische Mathematik [Numerical mathematics]. 1976;26(3):227–254. doi:10.1007/BF01395944
Musah M, Azeh Y, Mathew J, Umar M, Abdulhamid, Z, Muhammad A. Adsorption Kinetics and Isotherm Models: A review. CaJoST.2022;4(1):20–26. doi:10.4314/cajost.v4i1.3
Kreuzer HJ. Kinetics of adsorption, desorption and reactions at surfaces. In Rocca M, Rahman TS, Vattuone L. Springer Handbook of Surface Science. 1st ed. Cham: Springer International Publishing; 2020:1035–1052. doi:10.1007/978-3-030-46906-1_31
Islam MA, Chowdhury MA, Mozumder MdSI, Uddin MdT. Langmuir adsorption kinetics in liquid media: Interface reaction model. ACS Omega. 2021;6(22):14481–14492. doi:10.1021/acsomega.1c01449
Yuh-Shan H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics. 2004;59(1):171–177. doi:10.1023/B:SCIE.0000013305.99473.cf
Belcaid A, Beakou BH, Bouhsina S, Anouar A. Insight into adsorptive removal of methylene blue, malachite green, and rhodamine B dyes by cassava peel biochar (Manihot esculenta Crantz) in single, binary, and ternary systems: Competitive adsorption study and theoretical calculations. Biomass Conver Bioref. 2022;12(1):1–22. doi:10.1007/s13399-022-02928-w
Gayathiri M, Pulingam T, Lee KT, Mohd Din AT, Kosugi A, Sudesh K. Sustainable oil palm trunk fibre based activated carbon for the adsorption of methylene blue. Sci Rep. 2023;13(1):22137–22151. doi:10.1038/s41598-023-49079-0
Saravanan A, Yaashikaa PR, Kumar PS, Yuvaraj D, Karishma S, Muthu CMM, Nasrin MRT, Sree GA, Karthik V, Natrayan L, Rangasamy G. Adsorption performance and modelling of malachite green dye removal from aqueous solution using sulphuric acid–modified Ipomoea pes caprae biomass. Biomass Conv Bioref. 2023;13(16):15227–15309. doi:10.1007/s13399-023-05067-y
Ho YS, Wase DAJ, Forster CF. Kinetic studies of competitive heavy metal adsorption by sphagnum peat. Environ Tech. 1996;17:71-77. doi:10.1080/09593331708616362
Plazinski W, Dziuba J, Rudzinski W. Modeling of sorption kinetics: The pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorpt. 2013;19(5):1055–1064. doi:10.1007/s10450-013-9529-0
Shahwan T. Sorption kinetics: Obtaining a pseudo-second order rate equation based on a mass balance approach. J Environ Chem Eng. 2014;2(2):1001–1006. doi:10.1016/j.jece.2014.03.020
Robati D. Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J Nanostruct Chem. 2013;3(1):55–61. doi:10.1186/2193-8865-3-55
Hung YT, Holloman K. Agricultural waste as a low-cost adsorbent. In Wang LK, Wang MHS, Hung YT. Integrated Natural Resources Research. 1st ed. Cham: Springer International Publishing. 2021;22:103–146. doi:10.1007/978-3-030-61002-9_4
Zeldowitsch J.Über den mechanismus der katalytischen oxydation von CO and MnO2. Acta Physicochemical URSS. 1934;1:364-449. Russian.
Vargas-Rodríguez MY, Obaya A, García-Petronilo EJ, Vargas-Rodríguez IG, Gómez-Cortés A, Tavizón G, Chávez-Carvayar AJ. Adsorption studies of aqueous solutions of methyl green for halloysite nanotubes: Kinetics, isotherms, and thermodynamic parameters. Am J Nanomater. 2021;9(1):1–11. doi:10.12691/ajn-9-1-1
Ritchie AG. Alternative to the elovich equation for the kinetics of adsorption of gases on solids. J Chem Soc. 1977;73(0):1650. doi:10.1039/f19777301650
Xu Z, Cai J, Pan B. Mathematically modeling fixed-bed adsorption in aqueous systems. J Zhejiang Univ Sci A. 2013;14(3):155–176. doi:10.1631/jzus.A1300029
Solangi ZA, Bhatti I, Qureshi K. A combined CFD-response surface methodology approach for simulation and optimization of arsenic removal in a fixed bed adsorption column. Processes. 2022;10(9):1730–1747. doi:10.3390/pr10091730
Piccin JS, Cadaval TRS, de Pinto LAA, Dotto GL. Adsorption Isotherms in Liquid Phase: Experimental, Modeling, and Interpretations. In Bonilla-Petriciolet A, Mendoza-Castillo DI, Reynel-Ávila HE. Adsorption Processes for Water Treatment and Purification. 1st eds. Cham: Springer International Publishing. 2017;19–51. doi:10.1007/978-3-319-58136-1_2
Amrhar O, El Gana L, Mobarak M. Calculation of adsorption isotherms by statistical physics models: A review. Environ Chem Lett. 2021;19(6):4519–4547. doi:10.1007/s10311-021-01279-8
Limousin G, Gaudet JP, Charlet L, Szenknect S, Barthes V, Krimissa M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl Geochem. 2007;22:249–274. doi:10.1016/j.apgeochem.2006.09.010
Grassi M, Kaykioglu G, Belgiorno V, Lofrano G. Emerging Compounds Removal from Wastewater. In Green Chemistry for Sustainability, 15–38. Haz Mater. 2012;136(3):791–799. doi:10.1007/978-94-007-3916-1
Tadros T. (2013). Adsorption Isotherm. In Tadros T. Encyclopedia of Colloid and Interface Science. 1st eds. Berlin Heidelberg: Springer. 2013. 202 p. doi:10.1007/978-3-642-20665-8_46
Abin-Bazaine A, Campos Trujillo A, Olmos-Marquez M. Adsorption Isotherms: Enlightenment of the Phenomenon of Adsorption. In M. Ince M, Kaplan OI. 1st eds. Wastewater Treatment. IntechOpen. 2022:1–16. doi:10.5772/intechopen.104260
Ayawei N, Ebelegi AN, Wankasi D. Modelling and interpretation of adsorption isotherms. J Chem. 2017;2017:1–11. doi:10.1155/2017/3039817
Erdogan FO. Freundlich, Langmuir, Temkin, DR, Harkins-Jura Isotherm Studies on the Adsorption of CO2 on Various Porous Adsorbents. Int J Chem React Eng. 2019;17(5):1542–1580. doi:10.1515/ijcre-2018-0134
Akinbiyi OA. Removal of Lead from Aqueous Solutions by Adsorption using Peat Moss[thesis]. Regina (Canada): University of Regina; 2000. 101 p.
Demirbas E, Kobya M, Konukman AES. Error analysis of equilibrium studies for the almond shell activated carbon adsorption of Cr(VI) from aqueous solutions. J Haz Mater. 2008;154:787–794. doi:10.1016/j.jhazmat.2007.10.094
Ajemba RO. Thermodynamics and kinetic modeling of the dissolution and adsorptive applications of Ukpor, Udi, and Nteje clays[dissertation]. Anambra (Nigeria). Nnamdi Azikiwe University, 2012. 368 p.
Hall KR, Egleton LC, Acrivos A, Vemeulen T. Pore and solid diffusion kinetics in fixed bed adsorption under constant pattern conditions. Indus Eng Chem Fund. 1966;5(2): 212–223. doi:10.1021/i160018a011
Hu K, Zhang Q, Liu Y, Thaika MA. A developed dual-site Langmuir model to represent the high-pressure methane adsorption and thermodynamic parameters in shale. Inter J Coal Sci Technol. 2023;10(1):59. doi:10.1007/s40789-023-00629-x
Lohrentz L, Bhaumik M, Brink HG. High-capacity adsorption of hexavalent chromium by a polyaniline-Ni(0) nanocomposite adsorbent: Expanding the Langmuir-Hinshelwood kinetic model. J Molec Liquids. 2023;389:1–14. doi:10.1016/j.molliq.2023.122931
Brandani S. On adsorption azeotropy and a classification based on the dual site Langmuir isotherm. Adsorpt. 2023;1–9. doi:10.1007/s10450-023-00430-x
Freundlich H. Over the adsorption in solution. J Phys Chem. 1906;57:385–470. doi:10.1515/zpch-1907-5723
Burke GM, Wurster DE, Buraphacheep V, Berg MJ, Veng‐Pedersen P, Schottelius DD. Model selection for the adsorption of phenobarbital. Pharm Res. 1991;8(2):228–231. doi:10.1023/a:1015800322286
Adamson AW, Gast AP. Physical chemistry of surfaces. New York:Wiley & Sons; 1997. 765 p.
Vigdorowitsch M, Pchelintsev A, Tsygankova L, Tanygina E. Freundlich Isotherm: An Adsorption Model Complete Framework. Appl Sci. 2021;11(17):8078. doi:10.3390/app11178078
Van der Bruggen B, Enrico D, Lidietta G. Freundlich Isotherm. In Drioli E, Giorno L. Encyclopedia of Membranes .1st eds. Berlin Heidelberg: Springer. 2016. 834–835. doi:10.1007/978-3-662-44324-8_254
Melvin SS, Abigail MEA, Chidambaram R. Isotherm modelling, kinetic study and optimization of batch parameters using response surface methodology for effective removal of Cr(VI) using fungal biomass. PLOS One. 2015;10(3):1–13. doi:10.1371/journal.pone.0116884
Khayyun TS, Mseer AH. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl Water Sci. 2019;9(8):1–8. doi:10.1007/s13201-019-1061-2
Temkin MJ, Pyzhev V. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochimica URSS. 1940;12:217–224.
Hansen JB. Kinetics of Ammonia Synthesis and Decomposition on Heterogeneous Catalysts. In A. Nielsen A, Ammonia. 1st eds. Berlin Heidelberg: Springer. 1995:149–190. doi:10.1007/978-3-642-79197-0_4
Khandelwal A, Narayanan N, Varghese E, Gupta S. Linear and nonlinear isotherm models and error analysis for the sorption of kresoxim-methyl in Agriultural Soils of India. Bull Environ Contam Toxicol. 2020;104(4):503–510. doi:10.1007/s00128-020-02803-2
Mabuza M, Premlall K, Daramola MO. Modelling and thermodynamic properties of pure CO2 and flue gas sorption data on South African coals using Langmuir, Freundlich, Temkin and extended Langmuir isotherm models. Int J Coal Sci Technol. 2022;99(1):1-15. doi:10.1007/s00128-020-02803-2
Harkins WD, Jura EJ. The decrease of free surface energy as a basis for the development of equations for adsorption isotherms; and the existence of two condensed phases in films on solids. J Chem Phys. 1944;12:112–113. doi:10.1063/1.1723913
Shanavas S, Kunju AS, Varghese HT, Panicker CY. Comparison of Langmuir and Harkins-Jura adsorption isotherms for the determination of surface area of solids. Orien J Chem. 2011;27(1):245–252. Available from: http://www.orientjchem.org/?p=24831
Kausar A, Bhatti HN, MacKinnon G. Equilibrium, kinetic and thermodynamic studies on the removal of U(VI) by low-cost agricultural waste. Colloids Surfaces B Biointerfaces. 2013;111:124–133. doi:10.1016/j.colsurfb.2013.05.028
Hutson ND, Yang RT. Theoretical basis for the Dubinin-Radushkevitch (D-R) adsorption isotherm equation. Adsorption. 1997;3(3):189–195. doi:10.1007/BF01650130
Saeidi N, Parvini M. Accuracy of Dubinin-Astakov and Dubinin-Raduchkevic adsorption isotherm models in evaluating micropore volume of bontonite. Period Polytech Chem Eng. 2015;60(2):123–129. doi:10.3311/PPch.8374
Nguyen C, Do DD. The Dubinin-Raduskevich equation and the underlying microscopic adsorption-desorption. Carbon. 2001;39:1327–1336. doi:10.1016/S0008-6223(00)00265-7
Hu Q, Zhang Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J Molec Liquids. 2019;277:646–648. doi:10.1016/j.molliq.2019.01.005
Amrutha, Jeppu G, Girish CR, Prabhu B, Mayer K. Multi-component adsorption isotherms: Review and modeling studies. Environ Processes. 2023;10(2):1–52. doi:10.1007/s40710-023-00631-0
Sheindorf C, Rehbun M, Sheintuch M. A Freundlich type multicomponent isotherm. J Coll Inter Sci. 1981;79:136–142. doi:10.1016/0021-9797(81)90056-4
Abdehagh N, Tezel FH, Thibault J. Multi-component adsorption modeling: Isotherms for ABE model solutions using activated carbon F-400. Adsorpt. 2016;22(3):357–370. doi:10.1007/s10450-016-9784-y
Hilbrandt I, Lehmann V, Zietzschmann F, Ruhl AS, Jekel M. Quantification and isotherm modelling of competitive phosphate and silicate adsorption ontomicro-sized grazular ferric hydroxide. RSC Adv. 2019:9(41):23642–23651. doi:10.1039/C9RA04865K
Sursala S. Adsorption-desorption characteristics of phenoxyacetic acids and chlorophenols in a volcanic soil [dissertation]. New Palmerston(New Zealand); 1994. 228 p.
Wu J, Xie Z, Guo K, Claesson O. Measurement and prediction of the adsorption of binary mixtures of organic vapours on activated carbon. Adsorp Sci Technol. 2001;19(9):737–749. doi:10.1260/0263617011494547
Zarzour M, Ensafi AA, Rezaei B. Preparation of activated carbon from organic fraction of municipal solid wastes by ZnCl2 activation method and use it for elimination of chromium(VI) from aqueous solutions. J Iran Chem Soc. 2014;11(4):1075–1083. doi:10.1007/s13738-013-0375-5
Subramaniam R, Ponnusamy SK. Novel adsorbent from agricultural waste (cashew nut shell) for methylene blue dye removal: Optimization by response surface methodology. Water Res Indus. 2015;11:64–70. doi:10.1016/j.wri.2015.07.002
AlOthman Z, Habila M, Ali R. Preparation of activated carbon using the copyrolysis of agricultural and municipal solid wastes at a low carbonization temperature. Carbon. 2011;24:67–72. doi:10.13140/2.1.1478.2401
Kang C, Shang D, Yang T, Zhu L, Liu F, Wang N, Tian T. Preparation of corn stalk-walnut shell mix-based activated carbon and its adsorption of malachite green. Chem Res Chin Univ. 2018;34(6):1014–1019. doi:10.1007/s40242-018-8045-x
Haki AM, Imgharn A, Aarab N, Hsini A, Essekri A, Laabd M, El-Jazouli H, Elamine M, Lakhmiri R, Albourine A. Efficient removal of crystal violet dye from aqueous solutions using sodium hydroxide- modified avocado shells: Kinetics and isotherms modeling. Water Sci Technol. 2022;85(1):433–448. doi:10.2166/wst.2021.451
Khang DS, Hai TD, Thi TD, Tuan PD. Dye removal using cashew nut shell activated carbon. Viet J Chem. 2020;58(6):832–840. doi:10.1002/vjch.202000096
Rocha PD, Franca AS, Oliveira LS. Batch and column studies of phenol adsorption by an activated carbon based on acid treatment of corn cobs. Int J Eng Tech. 2015;7(6):459–464. doi:10.7763/IJET.2015.V7.837
Nazari G, Abolghasemi H, Esmaieli M, Assar M. Theoretical and experimental study of cephalex in batch adsorption dynamics using walnut shell-based activated carbon. Des Wat Treat. 2016;57(56):27339–27348. doi:10.1080/19443994.2016.1172029
Menkiti MC, Aneke MC, Ejikeme PM, Onukwuli OD, Menkiti NU. Adsorptive treatment of brewery effluent using activated Chrysophyllum albidium seed shell carbon. SpringerPlus. 2014;3(213):1–19. doi:10.1186/2193-1801-3-213
Sellaoui L, Yazidi A, Taamalli S, Bonilla-Petriciolet A, Louis F, El Bakali A, Badawi M, Lima EC, Lima DR, Chen Z. Adsorption of 3-aminophenol and resorcinol on avocado seed activated carbon: Mathematical modelling, thermodynamic study and description of adsorbent performance. J Molec Liquids. 2021;342:1–7. doi:10.1016/j.molliq.2021.116952
Rahman MM, Adil M, Yusof AM, Kamaruzzaman YB, Ansary RH. Removal of heavy metal ions with acid activated carbons derived from oil palm and coconut shells. Mater. 2014;7(5):3634–3650. doi:10.3390/ma7053634
Erhayem M, Al-Tohami F, Mohamed R, Ahmida K. Isotherm, kinetic and thermodynamic studies for the sorption of mercury (II) onto activated carbon from Rosmarinus officinalis Leaves. Am J Anal Chem. 2015;6:1-10. doi:10.4236/ajac.2015.61001
Maheshwari U, Mathesan B, Gupta S. Efficient adsorbent for simultaneous removal of Cu(II), Zn(II) and Cr(VI): Kinetic, thermodynamics and mass transfer mechanism. Proc Safety Environ Pro. 2015;98:198–210. doi:10.1016/j.psep.2015.07.010
Salman T, Aydın TF, Turan G, Ardalı Y. Removal of lead (II) from aqueous solution by batch adsorption on various inexpensive adsorbents using experimental design. Des Wat Treat. 2014;56:1–10. doi:10.1080/19443994.2014.951073
El-Naggar AH, Alzhrani AKR, Ahmad M, Usman ARA, Mohan D, Ok YS, Al-Wabel MI. Preparation of activated and non-activated carbon from conocarpus pruning waste as low-cost adsorbent for removal of heavy metal ions from aqueous solution. BioResources. 2016;11(1):1092–1107. doi:10.15376/biores.11.1.1092-1107
Mise S, Patil TN. Adsorption studies of chromium(VI) on activated carbon derived from mangifera indica (mango) seed shell. J Inst of Eng (India) Ser A. 2015;96(3):237–247. doi:10.1007/s40030-015-0124-0
Siripatana C, Khuenpetch A, Phromrak R, Saengngoen W, Nuithitikul K. Kinetic study of adsorption of lead (II) Ions onto cashew nut shells. ARPN J Eng Appl Sci. 2017;2(7):1819–1824. doi:10.3923/jeasci.2017.1819.1824
Nuithitikul K, Phromrak R, Saengngoen W. Utilization of chemically treated cashew-nutshell as potential adsorbent for removal of Pb(II) ions from aqueous solution. Sci Rep. 2020;10(1): 3343–3357. doi:10.1038/s41598-020-60161-9
Deokar SK, Mandavgane SA, Kulkarni BD. Adsorptive removal of 2,4-dichlorophenoxyacetic acid from aqueous solution using bagasse fly ash as adsorbent in batch and packed-bed techniques. Clean Tech Environ Pol. 2016;17:1–13. doi:10.1007/s10098-016-1124-0
Chatterjee A, Schiewer S. Biosorption of cadmium(II) ions by citrus peels in a packed bed column: Effect of process parameters and comparison of different breakthrough curve models. Clean-Soil Air Water. 2011;239(9):874–881. doi:10.1002/clen.201000482
Calero M, Blázquez G, Ronda A, Álvarez AE, Martín-Lara, M. Á. Biosorption of Cu2+ in a packed bed column by almond shell : Optimization of process variables. Des Water Treat. 2013;51(2):1954–1964. doi:10.1080/19443994.2012.715167
López-Cervantes J, Sánchez-Machado DI, Sánchez-Duarte RG, Correa-Murrieta MA. Study of a fixed-bed column in the adsorption of an azo dye from an aqueous medium using a chitosan–glutaraldehyde biosorbent. Adsorp Sci Technol. 2018;36(1–2):215–232. doi:10.1177/0263617416688021
Bohart GS, Adams EQ. Some aspects of the behaviour of the charcoal with respect to chlorine. J Am Chem Soc. 1920;42:523–544. doi:10.1021/ja01448a018
Rout PR, Dash RR, Bhunia P. Modelling and packed bed column studies on adsorptive removal of phosphate from aqueous solutions by a mixture of ground burnt patties and red soil. Adv Environ Res. 2014;3(3):231–251. doi:10.12989/aer.2014.3.3.231
Ghribi A, Chlendi M. Modeling of fixed bed adsorption: Application to the adsorption of an organic dye. Asian J Tex. 2011;1(4):161–171. doi:10.3923/ajt.2011.161.171
El-Naas MH, Alhaija MA, Al-Zuhair S. Evaluation of an activated carbon packed bed for the adsorption of phenols from petroleum refinery wastewater. Environ Sci PollUT Res. 2017;24(8):7511–7520. doi:10.1007/s11356-017-8469-8
Yoon YH, Nelson JN. Application of gas adsorption kinetics I: A theoretical model for respirator cartridge service life. The Amer Indus Hyg Assoc J. 1984;45(8):509–516. doi:10.1080/15298668491400197
Kavak, D, Öztürk N. Adsorption of boron from aqueous solution by sepirolite: II. Column studies. II. Illuslrararasi Bor Sempozyumu. 2004;23-25:495–500.
Yan G, Viraraghavan T, Chen M. A new model for heavy metal removal in a biosorption column. Ads Sci Technol. 2001;19(1):25–43. doi:10.1260/026361701149395
Kumar SR, Vijayaraghavan K, Thilakavathi M, Iyer PVR, Velan M. Seaweeds for the remedition of wastewaters contaminated with zinc (II) ions. J Haz Mater. 2006;136(3):791–799. doi:10.1016/j.jhazmat.2006.01.014
Araneda C, Basualto C, Sapag J, Tapia C, Cotoras D, Valenzuela F. Uptake of copper (II) ions from acidic aqueous solutions using a continuous column packed with microcapsules containing a β-hydroxyoximic compound. Chem Eng Res Des. 2011;89(12):2761–2769. doi:10.1016/j.cherd.2011.05.008
Khanh NH, Hoang NV. Adsorption process on fixed bed column in rich organic wastewater treatment experimental studies and numerical simulation. Vietnam J Mech. 2006;28(1):28–34. doi:10.15625/0866-7136/28/1/5476
Dinesha BL, Hiregoudar S, Nidoni U, Ramappa KT, Dandekar AT, Ganachari SV. Adsorption modelling and fixed-bed column study on milk processing industry wastewater treatment using chitosan zinc-oxide nano- adsorbent–coated sand filter bed. Environ Sci Pollut Res. 2022;30(13):37547–37569. doi:10.1007/s11356-022-24873-x
Mavinkattimath RG, Shetty Kodialbail V, Srinikethan, G. Continuous fixed-bed adsorption of reactive azo dye on activated red mud for wastewater treatment-Evaluation of column dynamics and design parameters. Environ Sci Pollut Res. 2023;30(19):57058–57075. doi:10.1007/s11356-023-26210-2
Mendes PAP, Rodrigues AE, Almeida JP, Silva JAC. Dynamics of a Fixed Bed Adsorption Column in the Kinetic Separation of Hexane Isomers in MOF ZIF-8. In Pinto AA, Zilberman D. (Eds.), Modeling, Dynamics, Optimization and Bioeconomics III.1st eds. Cham: Springer International Publishing. 2018;224:257–271. doi:10.1007/978-3-319-74086-7_12
Anisuzzaman SM, Bono A, Krishnaiah D, Tan YZ. A study on dynamic simulation of phenol adsorption in activated carbon packed bed column. J King Saud Uni Eng Sci.2014;30:1–30. doi:10.1016/j.jksues.2014.01.001
Arim AL, Neves K, Quina MJ, Gando-Ferreira LM. Experimental and mathematical modelling of Cr(III) sorption in fixed-bed column using modified pine bark. J Cleaner Product. 2018;183:272–281. doi:10.1016/j.jclepro.2018.02.094
Haroon H, Shah JA, Khan MS, Alam T, Khan R, Asad SA, Ali MA, Farooq G, Iqbal M, Bilal M. Activated carbon from a specific plant precursor biomass for hazardous Cr(VI) adsorption and recovery studies in batch and column reactors: Isotherm and kinetic modeling. J Water Process Eng. 2020;38:101–577. doi:10.1016/j.jwpe.2020.101577
Antil M, Singh S, Bhagat M, Vilvas V, Sundaramurthy S. Column optimization of adsorption and evaluation of bed parameters-based on removal of arsenite ion using rice husk. Environ Sci Pollut Res. 2022:1–15. doi:10.1007/s11356-022-20580-9
Warren D, Seider J, Seader JD, Lewin DR. Process Design Principles: Synthesis, Analysis, and Evaluation. New York: John Wiley and Sons; 1999. 766 p.
Kalogirou SA, Panteliou S, Dentsoras A. Artificial Neural Networks used for performance prediction of a thermosiphon solar water heater. Renewable Energy. 1999;18(1):87–99. doi:10.1016/S0960-1481(98)00787-3
Nascimento CAO, Giudici R, Guardani R. Neural network based approach for optimization of industrial chemical processes. 2000;24:2303–2314. doi:10.1016/S0098-1354(00)00587-1
Ayoub MA, Almansour AO, Hassan AM. A novel formula for estimating oil compressibility below bubble point pressure using group method of data handling: A comparative approach. In SPE/IATMI Asia Pacific Oil and Gas Conference and Exhibition. 2019 Oct 29-31; Bali, Indonesia. p. 12. doi:10.2118/196446-MS
Stanley FJ. The GMDH Algorithm of Ivakhnenko. Am Stat. 1981;35(4):210–215. doi:10.2307/2683292
Madala HR, Ivakhneko OG. Inductive learning algorithms for complex systems modeling. GMDH book. Boca Raton USA: CRC Press. 1994. 373 p.
Voss, MS. The Group Method of Cartesian Programming: A New Methodology for Complex Adaptive Functional Networks[dissertation]. Wisconson (USA):Marquette University; 2002. 261 p.
Sahu JN, Acharya J, Meikap BC. Optimization of production conditions for activated carbons from tamarind wood by zinc chloride using response surface methodology. Biores Tech. 2010;101(6):1974–1982. doi:10.1016/j.biortech.2009.10.031
Betiku E, Osunleke AS, Odude VO, Bamimore A, Oladipo B, Okeleye AA, Ishola NB. Performance Evaluation of Adaptive Neuro-Fuzzy Inference system, Artificial Neural Network and Response Surface Methodology in Modeling Biodiesel Synthesis from Palm Kernel Oil by Transesterification. Biofuels. 2018. doi:10.1080/17597269.2018.1472980.
Bhowmik M, Kanmani M, Animesh D, Biswajit S. Sono-assisted rapid adsorption of anionic dye onto magnetic CaFe2O4/MnFe2O4 nanocomposite from aqua matrix. Powder Technol. 2019;354:496–504. doi:10.1016/j.powtec.2019.06.009
Razzaq L, Abbas MM, Miran S, Asghar S, Nawaz S, Soudagar MEM, Shaukat N, Veza I, Khalil S, Abdelrahman A. Response surface methodology and artificial neural networks-based yield optimization of biodiesel sourced from mixture of palm and cotton seed oil. Sustainab. 2022;14:6130. doi:10.3390/su14106130
Thoai DN, Tongurai C, Prasertsit K, Kumar A. Predictive capability evaluation of RSM and ANN in modeling and optimization of biodiesel production from palm (Elaeisguineensis) oil. Int J Appl Eng Res. 2018;13:7529–7540.
Farobie O, Hasanah N, Matsumura Y. Artificial neural network modeling to predict Biodiesel production in supercritical methanol and ethanol using spiral reactor. Proc Environ Sci. 2015;28:214–223. doi:10.1016/j.proenv.2015.07.028
Haryanto A, Saputra TW, Telaumbanua M, Gita AC. Application of artificial neural network to predict biodiesel yield from waste frying oil transesterification. Ind J Sci Technol. 2019;5:62–74. doi:10.17509/ijost.v5i1.23099
Mahfouz AB, Ali A, Crocker M, Ahmed A, Nasir R, Show PL. Neural-network-inspired correlation (N2IC) model for estimating biodiesel conversion in algal biodiesel units. Fermentation. 2023;9:47. doi:10.3390/fermentation9010047
DOI: https://doi.org/10.15826/chimtech.2024.11.2.02
Copyright (c) 2024 Karinate Valentine Okiy, Joseph Nwabanne Tagbo, Walter Peter Echeng

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







