Photoelectrochemical properties of Pt- and Ir-modified graphitic carbon nitride
Abstract
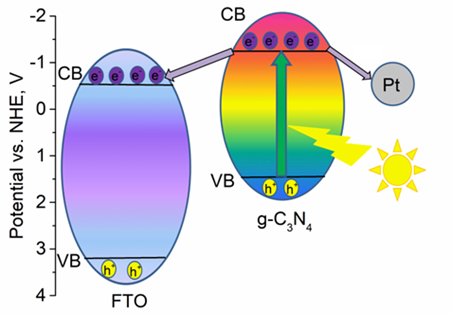
In this work, the photoelectrochemical properties of g-C3N4 modified with Pt, Ir and Ir/Pt bimetallic co-catalysts were studied. All prepared photoelectrodes were tested in a two-electrode cell by cyclic voltammetry, impedance spectroscopy, and the Mott-Schottky method. First, the optimal electrolyte (triethanolamine, NaCl, NaOH, Na2SO4) was selected. The highest photocurrents were recorded in 0.5 M Na2SO4. This electrolyte was used for the subsequent tests. Second, the photoelectrodes loaded with the noble metals are studied. It was shown that in case of monometallic co-catalysts, the deposition of noble metal is accompanied by the decrease of the short-circuit current density and the growth of open-circuit voltage. The simultaneous presence of bimetallic co-catalysts can significantly affect the semiconductor electron structure and photogalvanic properties. Some correlations between the short-circuit current density and the oxidation state of the noble metals were found. A linear correlation between Pt0/Pt0+Pt2+ and Jsc was observed. It was also shown that the presence of iridium in Ir3+ form favors the photocurrent generation. The highest values of the photocurrent were obtained for g-C3N4 and were equal to 0.57 mA/cm2.
Keywords
Full Text:
PDFReferences
Dada M, Popoola P. Recent advances in solar photovoltaic materials and systems for energy storage applications: a review. Beni-Suef Univ J Basic Appl Sci. 2023;12(66). doi:10.1186/s43088-023-00405-5
Wen J, Xie J, Chen X, Li X. A review on g-C3N4-based photocatalysts. Appl Surf Sci. 2017;391:72–123. doi:10.1016/j.apsusc.2016.07.030.
Qin DD, Quan JJ, Duan SF, San Martin J, Lin Y, Zhu X, Yao XQ, Su JZ, Rodríguez-Gutiérrez I, Tao CL, Yan Y. High-performance photoelectrochemical water oxidation with phosphorus-doped and metal phosphide cocatalyst-modified g-C3N4 formation through gas treatment. ChemSusChem. 2019;21;12(4):898–907. doi:10.1002/cssc.201802382
Amedlous A, Majdoub M, Amaterz E, Anfar Z, Benlhachemi A. Synergistic effect of g-C3N4 nanosheets/Ag3PO4 microcubes as efficient n-p-type heterostructure based photoanode for photoelectrocatalytic dye degradation. J Photochem Photobio A Chem. 2021;409:113127. doi:10.1016/j.jphotochem.2020.113127
Zhuang H, Lin J, Xu M, Xu W, Liu X. Construction of g-C3N4-based photoelectrodes towards photoelectrochemical water splitting: A review. J. Alloys Compd. 2023;969:172302. doi:10.1016/j.jallcom.2023.172302
Xiao J, Zhang X, Li Y. A ternary g-C3N4/Pt/ZnO photoanode for efficient photoelectrochemical water splitting. Int J Hydrog Energy. 2015;40(30):9080–9087. doi10.1016/j.ijhydene.2015.05.122.
Li W, Chu X-S, Wang F, Dang Y-Y, Liu X-Y, Wang H-C, Wang C. Enhanced cocatalyst-support interaction and promoted electron transfer of 3D porous g-C3N4/GO-M (Au, Pd, Pt) composite catalysts for hydrogen evolution. Appl Catal B Environm. 2021;288:120034. doi:10.1016/j.apcatb.2021.120034
Li H, Xia Z, Chen J, Lei L, Xing J. Constructing ternary CdS/reduced graphene oxide/TiO2 nanotube arrays hybrids for enhanced visible-light-driven photoelectrochemical and photocatalytic activity. Appl Catal B: Environm. 2015;168–169:105–113. doi:10.1016/j.apcatb.2014.12.029
Liang S, Xia Y, Zhu S, Zheng S, He Y, Bi J, Liu M, Wu L. Au and Pt co-loaded g-C3N4 nanosheets for enhanced photocatalytic hydrogen production under visible light irradiation. Appl Surf Sci. 2015;358:304–312. doi:10.1016/j.apsusc.2015.08.035
Bu Y, Chen Z, Li W. Using electrochemical methods to study the promotion mechanism of the photoelectric conversion performance of Ag-modified mesoporous g-C3N4 heterojunction material. Appl Catal BEnvironm. 2014;144:622–630. doi:10.1016/j.apcatb.2013.07.066
Markovskaya DV, Zhurenok AV, Kozlova EA. Rate of photocatalytic hydrogen evolution and photovoltaic characteristics as a function of the nature and concentration of the electrolyte. Russ J Phys Chem A. 2022:96(5):1093–1098.doi:10.1134/S003602442205020X
Wang W, Kou X, Li T, Zhao R, Su Y. Tunable heptazine/triazine feature of nitrogen deficient graphitic carbon nitride for electronic modulation and boosting photocatalytic hydrogen evolution. J Photochem Photobiol A Chem. 2023;435:114308. doi:10.1016/j.jphotochem.2022.114308
Yang H, Sun S, Duan R, Yang B, Yang M, Qi X, Cai C, Yun D, Yang Q, Cui J. Mechanism insight into enhanced photocatalytic hydrogen production by nitrogen vacancy-induced creating built-in electric field in porous graphitic carbon nitride nanosheets. Appl Surf Sci. 2023;631:157544. doi:10.1016/j.apsusc.2023.157544
Ruan X, Wang Z, Wei Z, Zhang H, Zhang L, Zhao X, Singh DJ, Zhao J, Cui X, Zheng W. Electron cloud density localized graphitic carbon nitride with enhanced optical absorption and carrier separation towards photocatalytic hydrogen evolution. Appl Surf Sci. 2022;601:154294. doi:10.1016/j.apsusc.2022.154294
Sherryna A, Tahir M, Yamani Z, Alias H. 2D/2D NiAl LDH integrated graphitic carbon nitride with robust interfacial contact for driving photocatalytic hydrogen production. Mater Today: Proceedings. 2023. doi:10.1016/j.matpr.2023.08.133
Shi J, Wang H, Nie J, Yang T, Ju C, Pu K, Shi J, Zhao T, Li H, Xue J. Alkali-assisted engineering of ultrathin graphite phase carbon nitride nanosheets with carbon vacancy and cyano group for significantly promoting photocatalytic hydrogen peroxide generation under visible light: Fast electron transfer channel. J Colloid Interf Sci. 2023;643:47–61. doi:10.1016/j.jcis.2023.03.209
Chen Y, Lei L, Gong Y, Wang H, Fan H, Wang W. Enhanced electron delocalization on pyrimidine doped graphitic carbon nitride for boosting photocatalytic hydrogen evolution. Int J Hydrog Energy. 2024;51A:1058–1068. doi:10.1016/j.ijhydene.2023.07.147
Xu M, Meng D, Yousaf AB, Ruan X, Cui X. Superior hydrophilic porous graphitic carbon nitride for enhanced photocatalytic hydrogen evolution. Mater Lett. 2023;350:134888. doi:10.1016/j.matlet.2023.134888
Baranowska D, Mijowska E, Zielinska B. Promotion of photocatalytic hydrogen evolution induced by graphitic carbon nitride transformation from 2D flakes to 1D nanowires. Mater Res Bull. 2023;163:112210. doi:10.1016/j.materresbull.2023.112210
Yang Y, Li S, Mao Y, Dang Y, Jiao Z, Xu K. Post-functionalization of graphitic carbon nitride for highly efficient photocatalytic hydrogen evolution. J Fuel Chem Technol. 2023;51(2):205–214. doi:10.1016/S1872-5813(22)60036-7
Li J, Peng H, Luo B, Cao J, Ma L, Jing D. The enhanced photocatalytic and photothermal effects of Ti3C2 Mxene quantum dot/macroscopic porous graphitic carbon nitride heterojunction for Hydrogen Production. J Colloid Interface Sci. 2023;641:309–318. doi:10.1016/j.jcis.2023.03.015.
Gao J, Li M, Chen H, Guo L, Li Z, Wang X. Microstructure regulation of graphitic carbon nitride nanotubes via quick thermal polymerization process for photocatalytic hydrogen evolution. J Photochem Photobiol A Chem. 2023;441:114747. doi:10.1016/j.jphotochem.2023.114747
Wang T, Wan T, He S, Wang J, Yu M, Jia Y, Tang Q. Facile fabrication of graphitic carbon nitride by solvothermal method with hierarchical structure and high visible light photocatalytic activity. J Taiwan Inst Chem E. 2023;145:104773. doi:10.1016/j.jtice.2023.104773
Wei J, Zhao R, Luo D, Lu X, Dong W, Huang Y, Cheng X, Ni Y. Atomically precise Ni6(SC2H4Ph)12 nanoclusters on graphitic carbon nitride nanosheets for boosting photocatalytic hydrogen evolution. J Colloid Interf Sci. 2023;631(A):212–221. doi:10.1016/j.jcis.2022.11.010
Baranowska D, Zielinkiewicz K, Kedzierski T, Mijowska E, Zielinska B. Heterostructure based on exfoliated graphitic carbon nitride coated by porous carbon for photocatalytic H2 evolution. Int J Hydrog Energy. 2022;47(84):35666–35679. doi:10.1016/j.ijhydene.2022.08.15
Yu Z, Guan C, Yue X, Xiang Q. Infiltration of C-ring into crystalline carbon nitride S-scheme homojunction for photocatalytic hydrogen evolution. Chinese J Catal. 2023;50:361–371. doi:10.1016/S1872-2067(23)64448-1.
Lei L, Fan H, Jia Y, Wu X, Zhong Q, Wang W. Ultrafast charge-transfer at interfaces between 2D graphitic carbon nitride thin film and carbon fiber towards enhanced photocatalytic hydrogen evolution. Appl Surf Sci. 2022;606:154938. doi:10.1016/j.apsusc.2022.154938
Das B, Devi M, Deb S, Dhar SS. Boosting photocatalytic property of graphitic carbon nitride with metal complex fabrication for efficient degradation of organic pollutants. Chemosphere. 2023;323:138230. doi:10.1016/j.chemosphere.2023.138230
Cheng C, Shi J, Mao L, Dong C-L, Huang Y-C, Zong S, Liu J, Shen S, Guo L. Ultrathin porous graphitic carbon nitride from recrystallized precursor toward significantly enhanced photocatalytic water splitting. J Colloid Interf Sci. 2023;637:271–282. doi:10.1016/j.jcis.2023.01.098
Luo M, Jiang G, Yu M, Yan Y, Qin Z, Li Y, Zhang Q. Constructing crystalline homophase carbon nitride S-scheme heterojunctions for efficient photocatalytic hydrogen evolution. J Mater Sci Technol. 2023;161:220–232. doi:10.1016/j.jmst.2023.03.038
Chang X, Fan H, Zhu S, Lei L, Wu X, Feng C, Wang W, Ma L. Engineering doping and defect in graphitic carbon nitride by one-pot method for enhanced photocatalytic hydrogen evolution. Ceram Inter. 2023;49(4):6729–6738. doi:10.1016/j.ceramint.2022.10.151
Xu X, Feng X, Wang W, Song K, Ma D, Zhou Y, Shi Y-W. Construction of II-type and Z-scheme binding structure in P-doped graphitic carbon nitride loaded with ZnO and ZnTCPP boosting photocatalytic hydrogen evolution. J Colloid Interf Sci. 2023;651:669–677. doi:10.1016/j.jcis.2023.08.033
Wang T, Wan T, He S, Wang J, Yu M, Jia Y, Tang Q. Fabrication of structural defects and carboxyl groups on graphitic carbon nitride with enhanced visible light photocatalytic activity. J Environ Chem Eng. 2023;11(3):2213–3437. doi:10.1016/j.jece.2023.110050
Inoue T, Chuaicham C, Saito N, Ohtani B, Sasaki K. Z-scheme heterojunction of graphitic carbon nitride and calcium ferrite in converter slag for the photocatalytic imidacloprid degradation and hydrogen evolution. J Photochem Photobiol A Chem. 2023;440:114644. doi:10.1016/j.jphotochem.2023.114644
Markovskaya DV, Kozlova EA. Application of the Similarity Theory to Analysis of Photocatalytic Hydrogen Production and Photocurrent Generation. Chimica Techno Acta. 2023;10(2):202310203:1–21. doi:10.15826/chimtech.2023.10.2.03
Sidorenko ND, Topchiyan PA, Saraev AA, Gerasimov EY, Zhurenok AV, Vasilchenko DB, Kozlova EA. Bimetallic Pt-IrOx/g-C3N4 photocatalysts for the highly efficient overall water splitting under visible light. Catalysts. 2024;14(4):225. doi:10.3390/catal14040225
Markovskaya DV, Zhurenok AV, Cherepanova SV, Kozlova EA. Solid Solutions of CdS and ZnS: Comparing Photocatalytic Activity and Photocurrent Generation. Appl Surf Sci Adv 2021;4:100076:1–8. doi:10.1016/j.apsadv.2021.100076
Kamat PV, Tvrdy K, Baker DR, Padich JG. Beyong photovoltaics: semiconductor nanoarchitectures for liquid-junction solar cells. Chem Rev. 2010;110:6664–6688. doi:10.1016/j.mssp.2021.105717.
Baudys M, Paušová S, Praus P, Brezová V, Dvoranová D, Barbieriková Z, Krýsa J. Graphitic Carbon Nitride for Photocatalytic Air Treatment. Mater. 2020;13(13):3038. doi:10.3390/ma13133038
Ofuonye B, Lee J, Yan M, Sun C, Zuo J-M, Adesida I. Electrical and microstructural properties of thermally annealed Ni/Au and Ni/Pt/Au Schottky contacts on AlGaN/GaN heterostructures. Semiconductor Sci Technol. 2014;29(9):095005. doi:10.1088/0268-1242/29/9/095005
Fei X, Zhang L, Yu J, Zhu B. DFT Study on Regulating the Electronic Structure and CO2 Reduction Reaction in BiOBr/Sulphur-Doped G-C3N4 S-Scheme Heterojunctions. Nanotechnology for Energy Applications. 2021; 3:698351. doi:10.3389/fnano.2021.698351
Kann S, Takemoto S, Kaneko K, Takahashi I, Sugimoto M, Shinohe T, Fujita S. Electrical properties of α-Ir2O3/α-Ga2O3 pn heterojunction diode and band alignment of the heterostructure. Appl Phys Lett. 2018;113:212104. doi:10.1063/1.5054054
Markovskaya D, Sidorenko N, Zhurenok A, Kozlova E. Studying Effects of External Conditions of Electrochemical Measurements on the Photoelectrochemical Properties of Semiconductors: Cyclic Voltammetry, Impedance Spectroscopy, and Mott-Schottky Method. Electrochem Mater Technol. 2023;2(2):20232013:1–14. doi:10.15826/elmattech.2023.2.013
Kaneko K, Fujita S. Novel p-type oxides with corundum structure for gallium oxide electronics. J Mater Res. 2022;37:651–659. doi:10.1557/s43578-021-00439-4
DOI: https://doi.org/10.15826/chimtech.2024.11.2.08
Copyright (c) 2024 Dina V. Markovskaya, Victoria A. Lomakina, Ekaterina A. Kozlova

This work is licensed under a Creative Commons Attribution 4.0 International License.
© Website Chimica Techno Acta, 2014–2024
ISSN 2411-1414 (Online)
This journal is licensed under a Creative Commons Attribution 4.0 International







