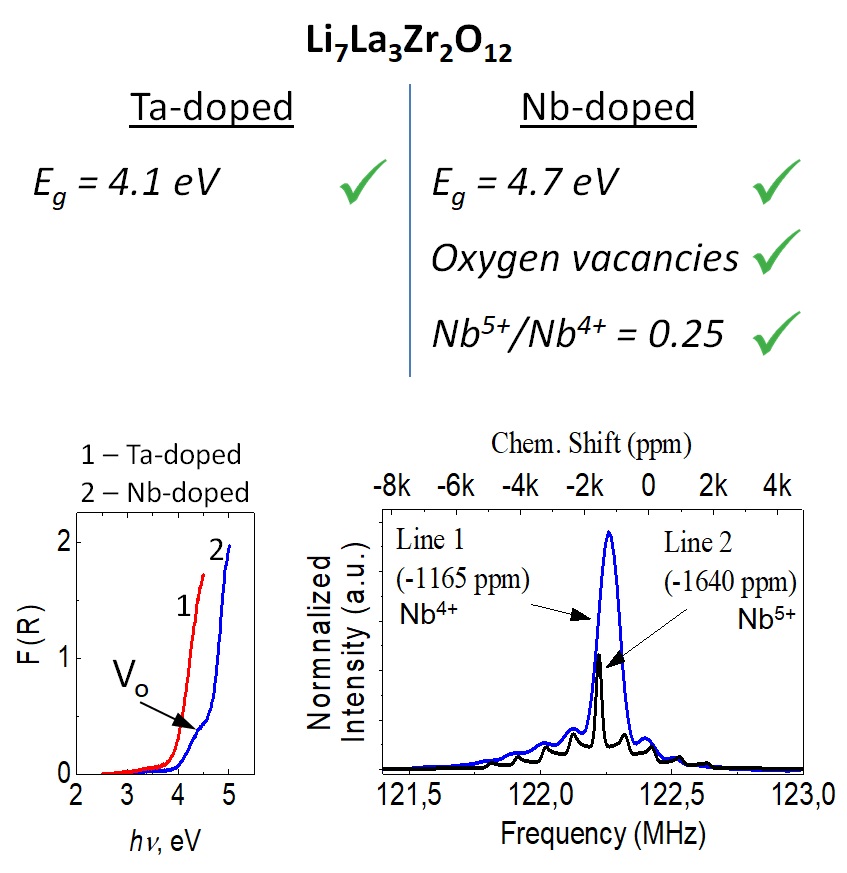
Features of electronic states in the vicinity of band gap and atomic structure of Ta- and Nb-doped Li7La3Zr2O12
Abstract
Keywords
Full Text:
PDFReferences
Sun C, Liu J, Gong Y, Wilkinson DP, Zhang J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy. 2017;33:363–386. doi:10.1016/j.nanoen.2017.01.028
Li C, Wang Z, He Z, Li Y, Mao J, Dai K, Yan C, Zheng J. An advance review of solid-state battery: Challenges, progress and prospects. Sustainable Mater Technol. 2021;29:e00297. doi:10.1016/j.susmat.2021.e00297
Ramakumar S, Deviannapoorani C, Dhivya L, Shankar LS, Murugan R. Lithium garnets: Synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog Mater Sci. 2017;88:325–411. doi:10.1016/j.pmatsci.2017.04.007
Geiger CA, Alekseev E, Lazic B, Fisch M, Armbruster T, Langner R, Fechtelkord M, Kim N, Pettke T, Weppner W. Crystal Chemistry and Stability of “Li7La3Zr2O12” Garnet: A Fast Lithium-Ion Conductor. Inorg Chem. 2011;50(3):1089–1097. doi:10.1021/ic101914e
Murugan R, Ramakumar S, Janani N. High conductive yttrium doped Li7La3Zr2O12 cubic lithium garnet. Electrochem Commun. 2011;13:1373–1375. doi:10.1016/j.elecom.2011.08.014
Huang M, Dumon A, Nan C. Effect of Si, In and Ge doping on high ionic conductivity of Li7La3Zr2O12. Electrochem Commun. 2012;21:62–64. doi:10.1016/j.elecom.2012.04.032
Janani N, Ramakumar S, Kannan S, Murugan R. Optimization of Lithium Content and Sintering Aid for Maximized Li+ Conductivity and Density in Ta-Doped Li7La3Zr2O12. J Am Ceram Soc. 2015;98(7):2039–2046. doi:10.1111/jace.13578
Ilina E, Lyalin E, Vlasov M, Kabanov A, Okhotnikov K, Sherstobitova E, Zobel M. Structural Features and the Li-Ion Diffusion Mechanism in Tantalum-Doped Li7La3Zr2O12 Solid Electrolytes. ACS Appl Energy Mater. 2022;5:2959−2967. doi:10.1021/acsaem.1c03632
Il’ina EA, Lyalin ED, Antonov BD, Pankratov AA. Lithium-Conducting Solid Electrolytes Synthesized by the Sol-Gel Method in the System Li7La3Zr2O12–Li5La3Nb2O12. Russ J Appl Chem. 2019;92(12):1657–1663. doi:10.1134/S107042721912005X
Vlasov MI, Zainullina VM, Korotin MA, Farlenkov AS, Ananyev MV. Effect of Proton Uptake on the Structure of Energy Levels in the Band-Gap of Sr-doped LaScO3: Diffuse Reflectance Spectroscopy and Coherent Potential Approximation Calculations. Phys Chem Chem Phys. 2019;21:7989–7995. doi:10.1039/C9CP00539K
Santosh KC, Longo RC, Xiong K, Cho K. Point defects in garnet-type solid electrolyte (c-Li7La3Zr2O12) for Li-ion batteries. Solid State Ionics. 2014;261:100–105. doi:10.1016/j.ssi.2014.04.021
Kubelka P. New Contributions to the Optics of Intensely Light-Scattering Materials. Part I. J Opt Soc Am. 1948;38:448–457. doi:10.1364/josa.38.000448
Sadykov AF, Gerashchenko AP, Piskunov YuV, Ogloblichev VV, Smol’nikov AG, Verkhovskii SV, Yakubovskii AYu, Tishchenko EA, Bush AA. Magnetic structure of low-dimensional LiCu2O2 multiferroic according to 63,65Cu and 7Li NMR studies. J Exp Theor Phys. 2012;115:666–672. doi:10.1134/S1063776112090105
Rettenwander D, Redhammer G, Preishuber-Pflügl F, Cheng L, Miara L, Wagner R, Welzl A, Suard E, Doeff MM, Wilkening M, Fleig J, Amthauer G. Structural and Electrochemical Consequences of Al and Ga Cosubstitution in Li7La3Zr2O12 Solid Electrolytes. Chem Mater. 2016; 28(7):2384–2392. doi:10.1021/acs.chemmater.6b00579
Tauc J. Optical properties and electronic structure of amorphous Ge and Si. Mater Res Bull. 1968;3(1):37–46. doi:10.1016/0025-5408(68)90023-8
Yifeng G, Liang X, Xinqi L, Jiang X. Electronic Structure and Electrochemical Properties of Garnet Type Li7La3Zr2O12 Solid Electrolytes Doped with Ta and Nb. Int J Electrochem Sci. 2022;17:220316. doi:10.20964/2022.03.03
Thompson T, Yu S, Williams L, Schmidt RD, Garcia-Mendez R, Wolfenstine J, Allen JL, Kioupakis E, Siegel DJ, Sakamoto J. Electrochemical Window of the Li-Ion Solid Electrolyte Li7La3Zr2O12. ACS Energy Lett. 2017;2:462–468. doi:10.1021/acsenergylett.6b00593
Tan J, Tiwari A. Characterization of Li7La3Zr2O12 Thin Films Prepared by Pulsed Laser Deposition. MRS Online Pros Library. 2012;1471:37–42. doi:10.1557/opl.2012.1266
Lapina OB, Khabibulin DF, Romanenko KV, Gan Z, Zuev MG, Krasil’nikov VN, Fedorov VE. 93Nb NMR chemical shift scale for niobia systems. Solid State Nucl Magn Reson. 2005;28:204–224. doi:10.1016/j.ssnmr.2005.09.003
Squires AG, Scanlon DO, Morgan BJ. Native Defects and Their Doping Response in the Lithium Solid Electrolyte Li7La3Zr2O12. Chem. Mater. 2020;32:1876−1886. doi:10.1021/acs.chemmater.9b04319
DOI: https://doi.org/10.15826/chimtech.2024.11.2.07
Copyright (c) 2024 Maxim I. Vlasov, Evgenii A. Surzhikov, Alexander Yu. Germov, Evgeniya A. Il'ina, Ilya A. Weinstein

This work is licensed under a Creative Commons Attribution 4.0 International License.
© Website Chimica Techno Acta, 2014–2024
ISSN 2411-1414 (Online)
This journal is licensed under a Creative Commons Attribution 4.0 International







