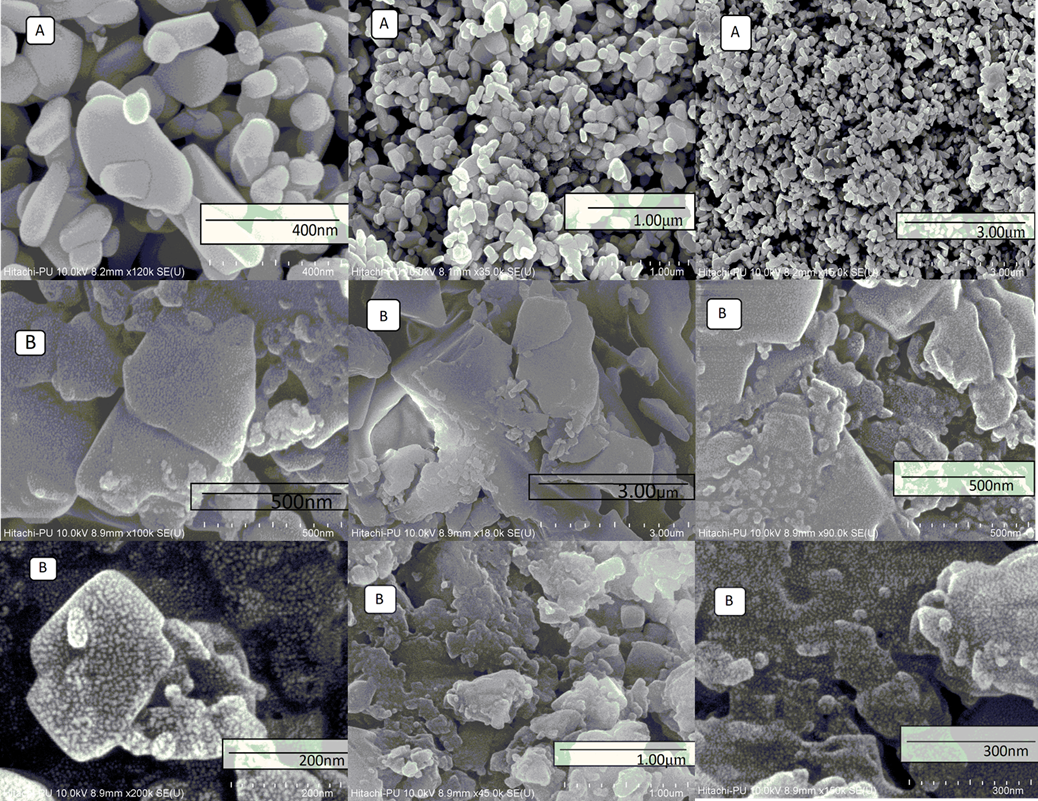
Investigation of the reaction of dimedone with aromatic aldehydes in the presence of copper oxide nanoparticles
Abstract
Keywords
Full Text:
PDFReferences
Clark JH, Macquarrie DJ. Environmentally friendly catalytic methods. Chem Soc Rev. 1996;25(5):303–310. doi:10.1039/CS9962500303
Sápi A, Rajkumar T, Kiss J, Kukovecz Á, Kónya Z, Somorjai GA. Metallic nanoparticles in heterogeneous catalysis. Catal Lett. 2021;151:2153–2175. doi:10.1007/s10562-020-03477-5
Astruc D. Introduction: nanoparticles in catalysis. Chem Rev. 2020;120(2):461–463. doi:10.1021/acs.chemrev.8b00696
Liu L, Corma A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem Rev. 2018;118(10):4981–5079. doi:10.1021/acs.chemrev.7b00776
Waris A, Din M, Ali A, Ali M, Afridi S, Baset A, Khan AU. A comprehensive review of green synthesis of copper oxide nanoparticles and their diverse biomedical applications. Inorg Chem Commun. 2021;123:108369. doi:10.1016/j.inoche.2020.108369
Vasantharaj S, Sathiyavimal S, Saravanan M, Senthilkumar P, Gnanasekaran K, Shanmugavel M, Manikandan E, Pugazhendhi A. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: characterization of antibacterial activity and dye degradation potential. J Photochem Photobiol B Biol. 2019;191:143–149. doi:10.1016/j.jphotobiol.2018.12.026
Prasad KP, Dhawale DS, Joseph S, Anand C, Wahab MA, Mano A, Sathish CI, Balasubramanian VV, Sivakumar T, Vinu A. Post-synthetic functionalization of mesoporous carbon electrodes with copper oxide nanoparticles for supercapacitor application. Microporous Mesoporous Mater. 2013;172:77–86. doi:10.1007/s11664-016-4587-1
Saravanakumar K, Sathiyaseelan A, Mariadoss A V, Xiaowen H, Wang MH. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int J Biol Macromol. 2020;153:207–214. doi:10.1016/j.ijbiomac.2020.02.250
Rajak R, Saraf M, Kumar P, Natarajan K, Mobin SM. Construction of a Cu-Based Metal–Organic Framework by Employing a Mixed-Ligand Strategy and Its Facile Conversion into Nanofibrous CuO for Electrochemical Energy Storage Applications. Inorg Chem. 2021;60(22):16986–16995. doi:10.1021/acs.inorgchem.1c02062
Singh P, Singh KR, Singh J, Singh RP. Biogenic synthesis of copper oxide nanoparticles: characterization and biosensing application. ECS Trans. 2022;107(1):20127. doi:10.1149/10701.20127ecst
Chang MH, Liu HS, Tai CY. Preparation of copper oxide nanoparticles and its application in nanofluid. Powder Technol. 2011;207(1–3):378–386. doi:10.1016/j.powtec.2010.11.022
Lasfargues M, Stead G, Amjad M, Ding Y, Wen D. In Situ production of copper oxide nanoparticles in a binary molten salt for concentrated solar power plant applications. Mater. 2017;10(5):537. doi:10.3390/ma10050537
Gnanavel V, Palanichamy V, Roopan SM. Biosynthesis and characterization of copper oxide nanoparticles and its anticancer activity on human colon cancer cell lines (HCT-116). J Photochem Photobiol B Biol. 2017;171:133–138. doi:10.1016/j.jphotobiol.2017.05.001
Chowdhury R, Khan A, Rashid MH. Green synthesis of CuO nanoparticles using Lantana camara flower extract and their potential catalytic activity towards the aza-Michael reaction. RSC Adv. 2020;10(24):14374–14385. doi:10.1039/D0RA01479F
Elahimehr Z, Nemati F, Elhampour A. Synthesis of a magnetic-based yolk-shell nano-reactor: A new class of monofunctional catalyst by Cu0-nanoparticles and its application as a highly effective and green catalyst for A3 coupling reaction. Arab J Chem. 2020;13(1):3372–3382. doi:10.1016/j.arabjc.2018.11.011
Gangaprasad D, Raj JP, Kiranmye T, Sadik SS, Elangovan J. A new paradigm of copper oxide nanoparticles catalyzed reactions: Synthesis of 1, 2, 3-triazoles through oxidative azide-olefin cycloaddition. RSC Adv. 2015;5(78):63473–63477. doi:10.1039/C5RA08693K
Zhao J, Niu Z, Fu H, Li Y. Ligand-free hydroboration of alkynes catalyzed by heterogeneous copper powder with high efficiency. Chem Commun. 2014;50(16):2058–2060. doi:10.1039/C3CC48670B
Fui CJ, Sarjadi MS, Sarkar SM, Rahman ML. Recent advancement of ullmann condensation coupling reaction in the formation of aryl-oxygen (CO) bonding by copper-mediated catalyst. Catalysts. 2020;10(10):1103. doi:10.3390/catal10101103
Halder M, Islam MM, Ansari Z, Ahammed S, Sen K, Islam SM. Biogenic nano-CuO-catalyzed facile C–N cross-coupling reactions: scope and mechanism. ACS Sustain Chem Eng. 2017;5(1):648–657. doi:10.1021/acssuschemeng.6b02013
Džambić A, Muratović S, Veljović E, Softić A, Dautović E, Šljivić M. Evaluation of Antioxidative, Antimicrobial and Cytotoxic Activity of the Synthetized Arylmethylenbis (3-Hydroxy-5,5-Dimethyl-2- Cyclohexen-1-One) Derivatives. Eur Chem Bull. 2020;9(9):285–290. doi:10.17628/ecb.2020.9.285-290
Amininia A, Pourshamsian, K Sadeghi B. Nano-ZnO Impregnated on Starch-A Highly Efficient Heterogeneous Bio-Based Catalyst for One-Pot Synthesis of Pyranopyrimidinone and Xanthene Derivatives as Potential Antibacterial Agents. Russ J Org Chem. 2020;56(7):1279–1288. doi:10.1134/S1070428020070234
Bortolot CS, da SM, Forezi L, Marra RK, Reis MI, Sá BV, Ghasemishahrestani Z, Sola-Penna M, Zancan P, Ferreira VF, de C da, Silva F. Design, synthesis and biological evaluation of 1H-1, 2, 3-triazole-linked-1H-dibenzo [b, h] xanthenes as inductors of ROS-mediated apoptosis in the breast cancer cell line MCF-7. Med Chem. 2019;15(2):119–129. doi:10.2174/1573406414666180524071409
Shagufta AI. Recent insight into the biological activities of synthetic xanthone derivatives. Eur J Med Chem. 2016;116:267–280. doi:10.1016/j.ejmech.2016.03.058
Miladiyah I, Jumina J, Haryana SM, Mustofa M. Biological activity, quantitative structure–activity relationship analysis, and molecular docking of xanthone derivatives as anticancer drugs. Drug Des Devel Ther. 2018;12:149–158. doi:10.2147/DDDT.S149973
Manikandan A, Sivakumar A, Nigam P S, Napoleon A. A Anticancer effects of novel tetrahydro-dimethyl-xanthene-diones. Anti-Cancer Agents Med Chem. 2020;20(7):909–916. doi:10.2174/1871520620666200318094138
Banerjee AG, Kothapalli LP, Sharma PA, Thomas AB, Nanda RK, Shrivastava SK, Khatanglekar VV. A facile microwave assisted one pot synthesis of novel xanthene derivatives as potential anti-inflammatory and analgesic agents. Arab J Chem. 2016;9:S480–S489. doi:10.1016/j.arabjc.2011.06.001
Li JT, Li YW, Song YL, Chen GF. Improved synthesis of 2, 2′-arylmethylene bis (3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) derivatives catalyzed by urea under ultrasound. Ultrason Sonochem. 2012;19(1):1–4. doi:10.1016/j.ultsonch.2011.05.001
Mandlimath TR, Umamahesh B, Sathiyanarayanan KI. Rapid one pot synthesis of xanthene derivatives by an efficient and reusable nano-ZnAl2O4–An insight into a new process. J Mol Catal A Chem. 2014;391:198–207. doi:10.1016/j.molcata.2014.04.030
Jin T S, Wang A Q, Ma H, Zhang J S. The reaction of aromatic aldehydes and 5,5-dimethyl-1,3-cyclohexanedione under solvent-free grinding conditions. Ind J Chem Sec B. 2006;45B(02):470–474. doi:10.1002/chin.200622038
Ilangovan A, Malayappasamy S, Muralidharan S. et al. A highly efficient green synthesis of 1, 8-dioxo-octahydroxanthenes. Chem Cent J. 2011;5:81. doi:10.1186/1752-153X-5-81
Suresh DK, Jagir SS. An efficient green protocol for the production of 1,8-dioxo-octahydroxanthenes in triethylammonium acetate (teaa) a recyclable inexpensive ionic liquid. Rasayan J Chem. 2009;2(4):937–940.
Hekmatshoar R, Kargar M, Mostashari A, Hashemi Z, Goli F. et al. copper octoate: a commercially available and cost-effective homogeneous catalyst for the facile synthesis of 2, 2’-arylmethylenebis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-ones). J Turk Chem Soc A Chem. 2015;2(4):1–11. doi:10.18596/jotcsa.44600
Gao H, Yang X, Tang X, Yin P and Mao Z. A Brief Synthesis of 2, 2’-Arylmethylene Bis (3-hydroxy-5, 5-dimethyl-2-cyclohexene-1-one) Catalyzed by TEAOH in Various Solvents. Curr Org Synth. 2019;16(7):1032–1039. doi:10.2174/1570179416666190723122816
Harichandran G, Parameswari P, David Amalraj S, Shanmugam P. Preparation of MnO2 nanoparticles and application in the Synthesis of 2, 2’-arylmethylene bis (3-hydroxy-5, 5- dimethyl-2-cyclohexene-1-one). Int J Innov Res Sci Eng. 2347–3207.
Gupta M, Gupta M. Copper (0) nanoparticles onto silica: A stable and facile catalyst for one-pot synthesis of 2,2'-arylmethylene bis(3-hydroxy-5, 5-dimethyl-2-cyclohexene-1-one) via cascade Knoevenagel/Michael reaction. J Chem Sci. 2016;128 (5):849–854. doi:10.1007/s12039-016-1080-6
Astarian J, Heydari R, Maghsoodlou MT, Yazdani-Elah-Abadi A. An efficient synthesis of 2, 2'-arylmethylene bis (3-hydroxy-5, 5-dimethyl-2-cyclohexene-1-one) derivatives using baker's yeast. Rev Roum Chim. 2019;64:259–264. doi:10.33224/rrch.2019.64.3.07
Vaid R, Gupta M, Kant R, Gupta VK. Domino Knoevenagel/Michael synthesis of 2, 2’-arylmethylenebis (3-hydroxy-5,5-dimethyl-2-cyclohexen-1-one) derivatives catalyzed by silica-diphenic acid and their single crystal X-ray analysis. J Chem Sci. 2016;128(6):967–976. doi:10.1007/s12039-016-1088-y
Ganesh Babu S, Karvembu R. CuO nanoparticles: a simple, effective, ligand free, and reusable heterogeneous catalyst for N-arylation of benzimidazole. Ind Eng Chem Res. 2011;50(16):9594–9600. doi:10.1021/ie200797e
Arora P, Kumar P, Tomar V, Sillanpää M, Joshi RK, Nemiwal M. C–N cross-coupling organic transformations catalyzed via copper oxide nanoparticles: A review (2016-present). Inorg Chem Commun. 2022;145:109982. doi:10.2139/ssrn.4127322
Arunkumar B, Jeyakumar S, Jothibas, MA. Strains Activity of CuO Nanoparticles using Copper Chloride Dihydrate by Sol-Gel Method. Asian J Chem. 2019;31(4):886–890. doi:10.14233/ajchem.2019.21820
Venyaminov SY, Prendergast FG. Water (H2O and D2O) molar absorptivity in the 1000-4000 cm-1 range and quantitative infrared spectroscopy of aqueous solutions. Anal Biochem. 1997;248:234–245. doi:10.1006/abio.1997.2136
42. Dhineshbabu NR, Rajendran V. Antibacterial activity of hybrid chitosan–cupric oxide nanoparticles on cotton fabric. IET nanobiotechnol. 2016;10(1):13-19. doi:10.1049/iet-nbt.2014.0073
Jin TS, Zhang JS, Wang AQ. & Li TS. Solid State Condensation Reactions Between Aldehydes and 5,5 Dimethyl 1,3cyclohexanedione by Grinding at Room Temperature Synth Commun. 2005;35(17):2339–2345. doi:10.1002/chin.200603148
DOI: https://doi.org/10.15826/chimtech.2024.11.3.03
Copyright (c) 2024 Ravina Meena, Harshita Sachdeva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







