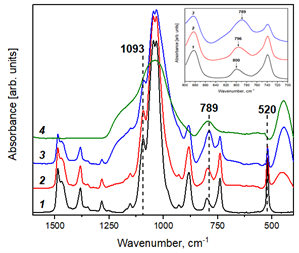
The effect of silicon dioxide on the structural, thermal and transport properties of an organic ionic plastic crystal (n-C4H9)4NBF4
Abstract
Keywords
Full Text:
PDFReferences
Campanella D, Belanger D, Paolella A. Beyond Garnets, Phosphates and Phosphosulfides Solid Electrolytes: New Ceramic Perspectives for All Solid Lithium Metal Batteries. J Power Sources. 2021;482:228949. doi:10.1016/j.jpowsour.2020.228949
Li S, Zhang S, Shen L, Liu Q, Ma J, Lv W, He Y, Yang Q. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv Sci. 2020;7(5):1903088. doi:10.1002/advs.20190308
Park KH, Bai Q, Kim DH, Oh DY, Zhu Y, Mo Y, Jung YS. Design Strategies, Practical Considerations, and New Solution Processes of Sulfide Solid Electrolytes for All-Solid-State Batteries. Adv. Energy Mater. 2018;8(18):1800035. doi:10.1002/aenm.201800035.
Ye T, Li L, Zhang Y. Recent Progress in Solid Electrolytes for Energy Storage Devices. Adv. Funct. Mater. 2020;30(29):2000077. doi:10.1002/adfm.202000077.
Kumaravel V, Bartlett J, Pillai SC. Solid Electrolytes for High-Temperature Stable Batteries and Supercapacitors. Adv Energy Mater. 2021;11(3):2002869. doi:10.1002/aenm.202002869
Chen S, Wen K, Fan J, Bando Y, Golberg D. Progress and Future Prospects of High-Voltage and High-Safety Electrolytes in Advanced Lithium Batteries: From Liquid to Solid Electrolytes. J. Mater. Chem. A. 2018;6(25):11631–11663. doi:10.1039/C8TA03358G
Hou M, Liang F, Chen K, Dai Y, Xue D. Challenges and Perspectives of NASICON-Type Solid Electrolytes for All-Solid-State Lithium Batteries. Nanotechnol. 2020;31(13):132003. doi:10.1088/1361-6528/ab5be7
Wu Z, Xie Z, Yoshida A, Wang Z, Hao X, Abudula A, Guan G. Utmost Limits of Various Solid Electrolytes in All-Solid-State Lithium Batteries: A Critical Review. Renew Sustain Energy Rev. 2019;109:367–385. doi:10.1016/j.rser.2019.04.035
Uvarov NF, Iskakova AA, Bulina NV, Gerasimov KB, Slobodyuk AB, Kavun VYa. Ion Conductivity of the Plastic Phase of the Organic Salt [(C4H9)4N]BF4. Russ J Electrochem. 2015;51(5):491–494. doi:10.1134/S102319351505016X
Abeysooriya S, Makhlooghiazad F, Chotard J-N, O’Dell LA, Pringle JM. Investigation of the Physicochemical Properties of Pyrrolidinium-Based Mixed Plastic Crystal Electrolytes. J Phys Chem C. 2023;127(25):12304–12320. doi:10.1021/acs.jpcc.3c02249
Pringle JM, Adebahr J, MacFarlane DR, Forsyth M. Unusual Phase Behaviour of the Organic Ionic Plastic Crystal N,N-Dimethylpyrrolidinium Tetrafluoroborate. Phys Chem Chem Phys. 2010;12(26):7234–7240. doi:10.1039/B925501J
Chae H, Lee Y-H, Yang M, Yoon W-J, Yoon DK, Jeong K-U, Song YH, Choi UH, Lee M. Interesting Phase Behaviors and Ion-Conducting Properties of Dicationic N -Alkylimidazolium Tetrafluoroborate Salts. RSC Adv. 2019;9(7):3972–3978. doi:10.1039/C8RA09208G
Matsumoto K, Harinaga U, Tanaka R, Koyama A, Hagiwara R, Tsunashima K. The Structural Classification of the Highly Disordered Crystal Phases of [Nn][BF4], [Nn][PF6], [Pn][BF4], and [Pn][PF6] Salts (Nn+ = Tetraalkylammonium and Pn+ = Tetraalkylphosphonium). Phys. Chem. Chem. Phys. 2014;16(43):23616–23626. doi:10.1039/C4CP03391D
Sourjah A, Kang CSM, Doherty CM, Acharya D, O’Dell LA, Pringle JM. New Organic Ionic Plastic Crystals Utilizing the Morpholinium Cation. Phys Chem Chem Phys. 2023;25(24):16469–16482. doi:10.1039/D3CP00759F
MacFarlane DR, Meakin P, Sun J, Amini N, Forsyth M. Pyrrolidinium Imides: A New Family of Molten Salts and Conductive Plastic Crystal Phases. J Phys Chem. B. 1999;103(20):4164–4170. doi:10.1021/jp984145s
Janikowski J, Razali MR, Forsyth CM, Nairn KM, Batten SR, MacFarlane DR, Pringle JM. Physical Properties and Structural Characterization of Ionic Liquids and Solid Electrolytes Utilizing the Carbamoylcyano(Nitroso)Methanide Anion. ChemPlusChem. 2013;78(6):486–497. doi:10.1002/cplu.201300068
Zhu H, MacFarlane DR, Pringle JM, Forsyth M. Organic Ionic Plastic Crystals as Solid-State Electrolytes. Trends Chem. 2019;1(1):126–140. doi:10.1016/j.trechm.2019.01.002
Pringle JM, Howlett PC, MacFarlane DR, Forsyth M. Organic Ionic Plastic Crystals: Recent Advances. J Mater Chem. 2010;20(11):2056. doi:10.1039/b920406
Jin L, Nairn KM, Forsyth CM, Seeber AJ, MacFarlane DR, Howlett PC, Forsyth M, Pringle JM. Structure and Transport Properties of a Plastic Crystal Ion Conductor: Diethyl(Methyl)(Isobutyl)Phosphonium Hexafluorophosphate. J Am Chem Soc. 2012;134(23):9688–9697. doi:10.1021/ja301175v
Uvarov NF, Asanbaeva NB, Ulihin AS, Mateyshina YG, Gerasimov KB. Thermal Properties and Ionic Conductivity of Tetra-n-Butylammonium Perchlorate. Cryst. 2022; 12(4):515. doi:10.3390/cryst12040515
Yunis R, W. Newbegin T, F. Hollenkamp A, M. Pringle J. Ionic Liquids and Plastic Crystals with a Symmetrical Pyrrolidinium Cation. Mater Chem Front. 2018;2(6):1207–1214. doi:10.1039/C8QM00016F
Guseva AF, Pestereva NN, Kuznetsov DK, Boyarshinova AA, Gardt VA. Conductivity of Composites MeWO4–Al2O3 (Me = Ca, Sr). Russ J Electrochem. 2023;59(4):284–290. doi:10.1134/S1023193523040079
Uvarov NF, Hairetdinov EF, Skobelev IV. Composite Solid Electrolytes MeNO3-Al2O3 (Me = Li, Na, K). Solid State Ion. 1996;86–88:577–580. doi:10.1016/0167-2738(96)00208-1
Kubataev ZYu, Gafurov MM, Rabadanov KSh, Amirov AM, Akhmedov MA, Kakagasanov MG. The Effect of the Nanosized Oxide Filler on the Structure and Conductivity of Composite (1–x)(LiClO4–NaClO4)–xAl2O3. Russ J Electrochem. 2023;59(8):598–603. doi:10.1134/S1023193523080050
Ponomareva VG, Lavrova GV, Simonova LG. Effect of SiO2 Morphology and Pores Size on the Proton Nanocomposite Electrolytes Properties. Solid State Ion. 1999;119(1–4):295–299. doi:10.1016/S0167-2738(98)00517-7
Ulihin AS, Uvarov NF. Electrochemical Properties of Composition Solid Electrolytes LiClO4-MgO. Russ J Electrochem. 2009;45(6):707–710. doi:10.1134/S1023193509060135
Uvarov NF, Ulihin AS, Slobodyuk AB, Kavun VY, Kirik SD. Nanocomposite Solid Electrolytes Based on Lithium Perchlorate. ECS Trans. 2008;11(31):9–17. doi:10.1149/1.2953501
Mateyshina Y, Uvarov N. The Effect of Oxide Additives on the Transport Properties of Cesium Nitrite. Solid State Ion. 2018;324:1–6. doi:10.1016/j.ssi.2018.05.017
Ulihin AS, Uvarov NF, Rabadanov KSh, Gafurov MM, Gerasimov KB. Thermal, Structural and Transport Properties of Composite Solid Electrolytes (1-x)(C4H9)4NBF4–xAl2O3. Solid State Ion. 2022;378:115889. doi:10.1016/j.ssi.2022.115889
Adebahr J, Ciccosillo N, Shekibi Y, Macfarlane D, Hill A, Forsyth M. The “Filler-Effect” in Organic Ionic Plastic Crystals: Enhanced Conductivity by the Addition of Nano-Sized TiO2. Solid State Ion. 2006;177(9–10):827–831. doi:10.1016/j.ssi.2006.02.022
Pringle JM, Shekibi Y, MacFarlane DR, Forsyth M. The Influence of Different Nanoparticles on a Range of Organic Ionic Plastic Crystals. Electrochim Acta. 2010;55(28):8847–8854. doi:10.1016/j.electacta.2010.08.027
Mateyshina Y, Stebnitskii I, Shivtsov D, Ilyina E, Ulihin A, Bukhtiyarov A, Uvarov N. Hybrid Nanocomposite Solid Electrolytes (n-C4H9)4NBF4–MgO. Int J Mo Sci. 2023;24(13):10949. doi:10.3390/ijms241310949
Mateyshina Y, Stebnitskii I, Uvarov N. Composite Solid Electrolytes (n-C4H9)4NBF4–Nanodiamonds. Solid State Ion. 2024;404:116419. doi:10.1016/j.ssi.2023.116419
Uvarov NF, Boldyrev VV. Size Effects in Chemistry of Heterogeneous Systems. Russ Chem Rev. 2001;70(4):265–284. doi:10.1070/RC2001v070n04ABEH000638
Uvarov NF, Vaněk P, Yuzyuk YuI, Železný V, Studnička V, Bokhonov BB, Dulepov VE, Petzelt J. Properties of Rubidium Nitrate in Ion-Conducting RbNO3-Al2O3 Nanocomposites. Solid State Ion. 1996;90(1):201–207. doi:10.1016/S0167-2738(96)00400-6
Stebnitsky IA, Uvarov NF, Mateyshina YuG. Synthesis and Study of the Physicochemical Properties of Composite Solid Electrolytes (C4H9)3CH3NBF4–Cnanodiamonds. Russ J Electrochem. 2024;60(1):18–24. doi:10.1134/S1023193524010105
Rabadanov KSh, Gafurov MM, Uvarov NF, Ulikhin AS. Temperature-Phase Dependence of the Vibration Spectrum and Orientation Mobility of the Tetrafluoroborate Ion in n-Bu4NBF4 Organic Salt. Phys Solid State. 2018;60(12):2593–2597. doi:10.1134/S1063783418120235
Uvarov NF. Composite Solid Electrolytes: Recent Advances and Design Strategies. J Solid State Electrochem. 2011;15(2):367–389. doi:10.1007/s10008-008-0739-4
DOI: https://doi.org/10.15826/chimtech.2024.11.3.07
Copyright (c) 2024 Ivan Stebnitskii, Yulia Mateyshina, Nikolai Uvarov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







