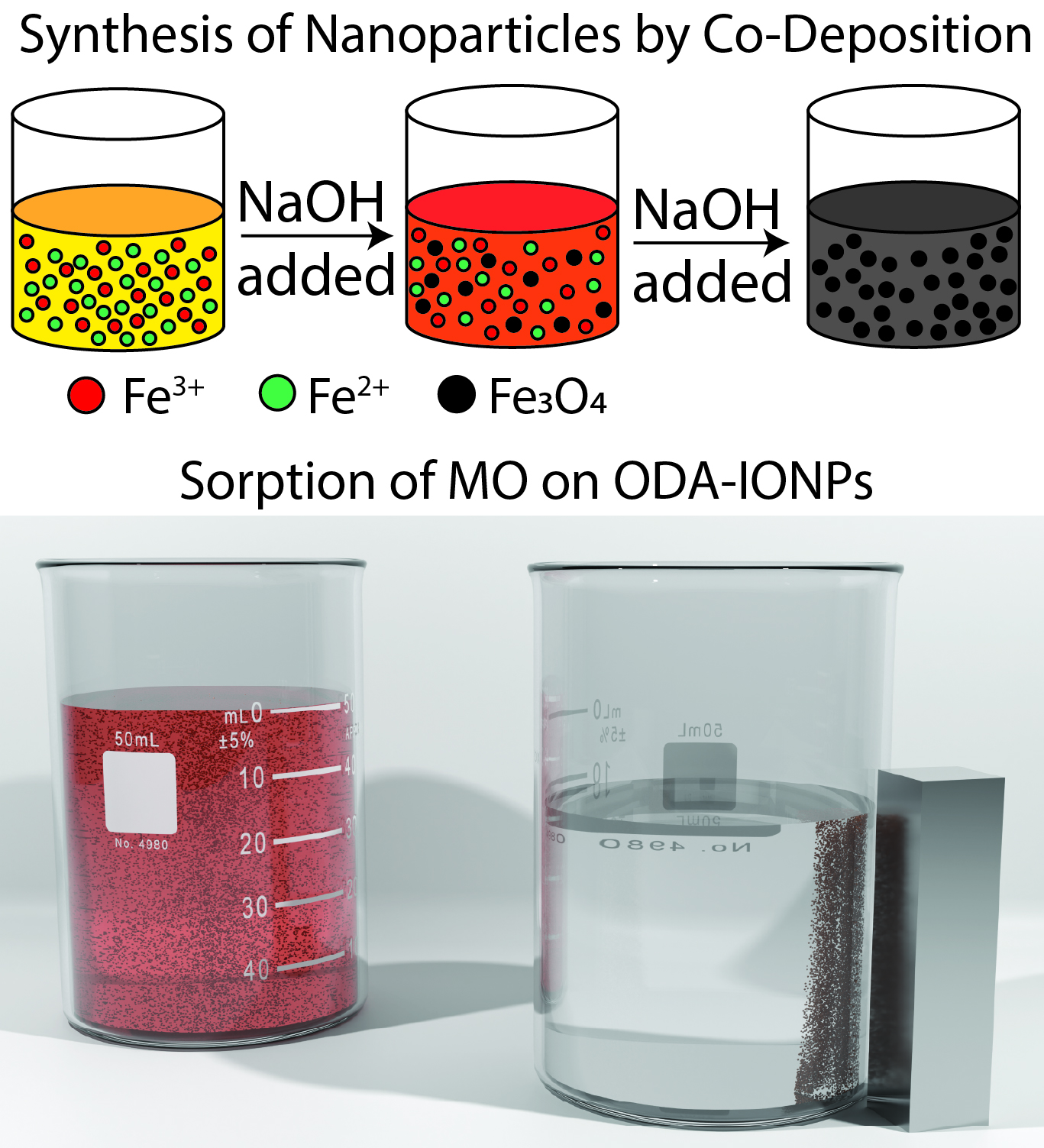
Methyl orange sorption on octadecylamine-modified iron oxide magnetic nanoparticles
Abstract
Keywords
Full Text:
PDFReferences
Lin J, Ye W, Xie M, Seo DH, Luo J, Wan Y, et al. Environmental impacts and remediation of dye-containing wastewater. Nat Rev Earth Environ 2023 411. 2023;4:785–803. doi:10.1038/s43017-023-00489-8
Ali K, Zeidan H, Amar B, Ali ( K, Zeidan H. Evaluation of the use of agricultural waste materials as low-cost and eco-friendly sorbents to remove dyes from water: a review. 2023 [cited 18 May 2024]. doi:10.5004/dwt.2023.29725
Al-Tohamy R, Ali SS, Li F, Okasha KM, Mahmoud YAG, Elsamahy T, et al. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol Environ Saf. 2022;231:113160. Medline:35026583 doi:10.1016/J.ECOENV.2021.113160
Jan S, Mishra AK, Bhat MA, Bhat MA, Jan AT. Pollutants in aquatic system: a frontier perspective of emerging threat and strategies to solve the crisis for safe drinking water. Environ Sci Pollut Res Int. 2023;30:113242–79. Medline:37864686 doi:10.1007/S11356-023-30302-4
Islam T, Repon MR, Islam T, Sarwar Z, Rahman MM. Impact of textile dyes on health and ecosystem: a review of structure, causes, and potential solutions. Environ Sci Pollut Res 2022 304. 2022;30:9207–42. Medline:36459315 doi:10.1007/S11356-022-24398-3
Sehar S, Rasool T, Syed HM, Mir MA, Naz I, Rehman A, et al. Recent advances in biodecolorization and biodegradation of environmental threatening textile finishing dyes. 3 Biotech. 2022;12:1–12. doi:10.1007/S13205-022-03247-7
Peramune D, Manatunga DC, Dassanayake RS, Premalal V, Liyanage RN, Gunathilake C, et al. Recent advances in biopolymer-based advanced oxidation processes for dye removal applications: A review. Environ Res. 2022;215:114242. Medline:36067842 doi:10.1016/J.ENVRES.2022.114242
Saad I, Ralha N, Abukhadra MR, Al Zoubi W, Ko YG. Recent advances in photocatalytic oxidation techniques for decontamination of water. J Water Process Eng. 2023;52:103572. doi:10.1016/J.JWPE.2023.103572
Solayman HM, Hossen MA, Abd Aziz A, Yahya NY, Leong KH, Sim LC, et al. Performance evaluation of dye wastewater treatment technologies: A review. J Environ Chem Eng. 2023;11:109610. doi:10.1016/J.JECE.2023.109610
Wang J, Guo X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J Hazard Mater. 2020;390:122156. Medline:32006847 doi:10.1016/J.JHAZMAT.2020.122156
Feng X, Peng D, Zhu J, Wang Y, Zhang Y. Recent advances of loose nanofiltration membranes for dye/salt separation. Sep Purif Technol. 2022;285:120228. doi:10.1016/J.SEPPUR.2021.120228
Cai L, Ying D, Liang X, Zhu M, Lin X, Xu Q, et al. A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem Eng J. 2021;410:128404. doi:10.1016/J.CEJ.2021.128404
Ahmed SF, Mofijur M, Nuzhat S, Chowdhury AT, Rafa N, Uddin MA, et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J Hazard Mater. 2021;416:125912. doi:10.1016/J.JHAZMAT.2021.125912
Khouni I, Marrot B, Amar R Ben. Treatment of reconstituted textile wastewater containing a reactive dye in an aerobic sequencing batch reactor using a novel bacterial consortium. Sep Purif Technol. 2012;87:110–9. doi:10.1016/J.SEPPUR.2011.11.030
Türgay O, Ersöz G, Atalay S, Forss J, Welander U. The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation. Sep Purif Technol. 2011;79:26–33. doi:10.1016/J.SEPPUR.2011.03.007
Lin X, Zhou Q, Xu H, Chen H, Xue G. Advances from conventional to biochar enhanced biotreatment of dyeing wastewater: A critical review. Sci Total Environ. 2024;907:167975. doi:10.1016/J.SCITOTENV.2023.167975
Kasbaji M, Mennani M, Oubenali M, Ait Benhamou A, Boussetta A, Ablouh EH, et al. Bio-based functionalized adsorptive polymers for sustainable water decontamination: A systematic review of challenges and real-world implementation. Environ Pollut. 2023;335:122349. doi:10.1016/J.ENVPOL.2023.122349
Yu JX, Chi RA, He ZY, Qi YF, Zhan G, Guo J. Combination of biosorption and photodegradation to remove methyl orange from aqueous solutions. Eng Life Sci. 2011;11:309–315. doi:10.1002/ELSC.201000158
Särkkä H, Bhatnagar A, Sillanpää M. Recent developments of electro-oxidation in water treatment — A review. J Electroanal Chem. 2015;754:46–56. doi:10.1016/J.JELECHEM.2015.06.016
Ondersma JW, Hamann TW. Recombination and redox couples in dye-sensitized solar cells. Coord Chem Rev. 2013;257:1533–43. doi:10.1016/J.CCR.2012.09.010
Al-Raad AA, Hanafiah MM. Removal of inorganic pollutants using electrocoagulation technology: A review of emerging applications and mechanisms. J Environ Manage. 2021;300:113696. doi:10.1016/j.jenvman.2021.113696
Aragaw TA, Bogale FM. Role of coagulation/flocculation as a pretreatment option to reduce colloidal/bio-colloidal fouling in tertiary filtration of textile wastewater: A review and future outlooks. Front Environ Sci. 2023;11:1142227. doi:10.3389/FENVS.2023.1142227/BIBTEX
Khan MD, Singh A, Khan MZ, Tabraiz S, Sheikh J. Current perspectives, recent advancements, and efficiencies of various dye-containing wastewater treatment technologies. J Water Process Eng. 2023;53:103579. doi:10.1016/J.JWPE.2023.103579
Islam M, Kumar S, Saxena N, Nafees A. Photocatalytic Degradation of Dyes Present in Industrial Effluents: A Review. ChemistrySelect. 2023;8:e202301048. doi:10.1002/SLCT.202301048
Ahsan A, Jamil F, Rashad MA, Hussain M, Inayat A, Akhter P, et al. Wastewater from the textile industry: Review of the technologies for wastewater treatment and reuse. Korean J Chem Eng 2023 409. 2023;40:2060–2081. doi:10.1007/S11814-023-1475-2
Ledakowicz S, Pázdzior K. Recent Achievements in Dyes Removal Focused on Advanced Oxidation Processes Integrated with Biological Methods. Mol 2021, Vol 26, Page 870. 2021;26:870. doi:10.3390/MOLECULES26040870
Darwish AAA, Rashad M, AL-Aoh HA. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dye Pigment. 2019;160:563–71. doi:10.1016/J.DYEPIG.2018.08.045
Jorge AMS, Athira KK, Alves MB, Gardas RL, Pereira JFB. Textile dyes effluents: A current scenario and the use of aqueous biphasic systems for the recovery of dyes. J Water Process Eng. 2023;55:104125. doi:10.1016/J.JWPE.2023.104125
Kumar N, Pandey A, Rosy, Sharma YC. A review on sustainable mesoporous activated carbon as adsorbent for efficient removal of hazardous dyes from industrial wastewater. J Water Process Eng. 2023;54:104054. doi:10.1016/J.JWPE.2023.104054
Zhou J, Tang C, Cheng B, Yu J, Jaroniec M. Rattle-type carbon-alumina core-shell spheres: Synthesis and application for adsorption of organic dyes. ACS Appl Mater Interfaces. 2012;4:2174–2179. doi:10.1021/AM300176K
Correa-Coyac D, Michtchenko A, Zacahua-Tlacuatl G, Cruz-Narváez Y, Castro-Arellano JJ, Sanpedro-Díaz M, et al. Adsorption and Photodegradation of Lanasol Yellow 4G in Aqueous Solution by Natural Zeolite Treated by CO2-Laser Radiation. Materials (Basel). 2023;16:4855. doi:10.3390/MA16134855
Ma Z, Zhang Q, Weng X, Mang C, Si L, Guan Z, et al. Fluoride ion adsorption from wastewater using magnesium(II), aluminum(III) and titanium(IV) modified natural zeolite: Kinetics, thermodynamics, and mechanistic aspects of adsorption. J Water Reuse Desalin. 2018;8:479–489. doi:10.2166/WRD.2017.037
Li CJ, Zhang YJ, Chen H, He PY, Meng Q. Development of porous and reusable geopolymer adsorbents for dye wastewater treatment. J Clean Prod. 2022;348:131278. doi:10.1016/J.JCLEPRO.2022.131278
Tariq R, Abatal M, Bassam A. Computational intelligence for empirical modeling and optimization of methylene blue adsorption phenomena using available local zeolites and clay of Morocco. J Clean Prod. 2022;370:133517. doi:10.1016/J.JCLEPRO.2022.133517
Shaban M, Abukhadra MR, Hamd A. Recycling of glass in synthesis of MCM-48 mesoporous silica as catalyst support for Ni2O3 photocatalyst for Congo red dye removal. Clean Technol Environ Policy. 2018;20:13–28. doi:10.1007/S10098-017-1447-5
López-Rodríguez D, Micó-Vicent B, Jordán-Núñez J, Bonet-Aracil M, Bou-Belda E. Uses of Nanoclays and Adsorbents for Dye Recovery: A Textile Industry Review. Appl Sci 2021, Vol 11, Page 11422. 2021;11:11422. doi:10.3390/APP112311422
Orooji Y, Han N, Nezafat Z, Shafiei N, Shen Z, Nasrollahzadeh M, et al. Valorisation of nuts biowaste: Prospects in sustainable bio(nano)catalysts and environmental applications. J Clean Prod. 2022;347:131220. doi:10.1016/J.JCLEPRO.2022.131220
Li H, Li M, Zheng F, Wang J, Chen L, Hu P, et al. Efficient removal of water pollutants by hierarchical porous zeolite-activated carbon prepared from coal gangue and bamboo. J Clean Prod. 2021;325:129322. doi:10.1016/J.JCLEPRO.2021.129322
Yuan N, Zhao A, Hu Z, Tan K, Zhang J. Preparation and application of porous materials from coal gasification slag for wastewater treatment: A review. Chemosphere. 2022;287:132227. doi:10.1016/J.CHEMOSPHERE.2021.132227
Chai Z, Liu B, Lv P, Bai Y, Wang J, Song X, et al. Recycling of coal gasification fine slag as ultra-high capacity adsorbents for the removal of Rhodamine B dye: Graded synthesis method, kinetics and adsorption mechanism. Fuel. 2023;333:126318. doi:10.1016/J.FUEL.2022.126318
Mohammad Hosseini N, Sheshmani S, Shahvelayati AS, Ahmadi R, Adhami F. Development and Characterization of Environmentally-Friendly Magnetically Graphene Oxide-Embedded Chitosan as a Recyclable Heterogeneous Photocatalyst. J Polym Environ. 2024;32:1952–1971. doi:10.1007/S10924-023-03117-0
Slama H Ben, Chenari Bouket A, Pourhassan Z, Alenezi FN, Silini A, Cherif-Silini H, et al. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl Sci. 2021;11. doi:10.3390/app11146255
Katheresan V, Kansedo J, Lau SY. Efficiency of various recent wastewater dye removal methods: A review. J Environ Chem Eng. 2018;6:4676–97. doi:10.1016/J.JECE.2018.06.060
Mohammed L, Gomaa HG, Ragab D, Zhu J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology. 2017;30:1–14. doi:10.1016/j.partic.2016.06.001
Ahmad T, Phul R, Khan H. Iron Oxide Nanoparticles: An Efficient Nano-catalyst. Curr Org Chem. 2019;23:994–1004. doi:10.2174/1385272823666190314153208
Adak L, Kundu D, Roy K, Saha M, Roy A. Reusable Iron/Iron Oxide-based Nanoparticles Catalyzed Organic Reactions. Curr Org Chem. 2022;26:399–417. doi:10.2174/1385272826666220209120545
Campos AFC, Michels-Brito PH, Da Silva FG, Gomes RC, Gomide G, Depeyrot J. Removal of direct yellow 12 from water using CTAB-coated core-shell bimagnetic nanoadsorbents. J Environ Chem Eng. 2019;7:103031. doi:10.1016/j.jece.2019.103031
Magomedov KE, Omelyanchik AS, Vorontsov SA, Čižmár E, Rodionova VV, Levada EV. SDS-Modified Iron Oxide Magnetic Nanoparticles for Removing of Methylene Blue from Aqueous Solution. Bull Russ Acad Sci Phys. 2023;87:720–727. doi:10.3103/S1062873823702027
Massart R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans Magn. 1981;17:1247–8. doi:10.1109/TMAG.1981.1061188
Omelyanchik A, da Silva FG, Gomide G, Kozenkov I, Depeyrot J, Aquino R, et al. Effect of citric acid on the morpho-structural and magnetic properties of ultrasmall iron oxide nanoparticles. J Alloys Compd. 2021;883:160779. doi:10.1016/J.JALLCOM.2021.160779
Omelyanchik A, Kamzin AS, Valiullin AA, Semenov VG, Vereshchagin SN, Volochaev M, et al. Iron oxide nanoparticles synthesized by a glycine-modified coprecipitation method: Structure and magnetic properties. Colloids Surfaces A Physicochem Eng Asp. 2022;647:129090. doi:10.1016/J.COLSURFA.2022.129090
Scano A, Cabras V, Pilloni M, Ennas G. Microemulsions: The Renaissance of Ferrite Nanoparticle Synthesis. J Nanosci Nanotechnol. 2019;19:4824–4838. doi:10.1166/JNN.2019.16876
Muscas G, Peddis D, Cobianchi M, Lascialfari A, Cannas C, Musinu A, et al. Magnetic Interactions Versus Magnetic Anisotropy in Spinel Ferrite Nanoparticles. IEEE Magn Lett. 2019;10. doi:10.1109/LMAG.2019.2956908
Santoyo Salazar J, Perez L, De Abril O, Truong Phuoc L, Ihiawakrim D, Vazquez M, et al. Magnetic iron oxide nanoparticles in 10-40 nm range: Composition in terms of magnetite/maghemite ratio and effect on the magnetic properties. Chem Mater. 2011;23:1379–86. doi:10.1021/CM103188A
Synthesis of Superparamagnetic Iron Oxide Nanoparticles: SWOT Analysis Towards Their Conjugation to Biomolecules for Molecular Recognition Applications. J Nanosci Nanotechnol. 2019;19. doi:10.1166/jnn.2019.16931
Andrade ÂL, Fabris JD, Ardisson JD, Valente MA, Ferreira JMF. Effect of Tetramethylammonium Hydroxide on Nucleation, Surface Modification and Growth of Magnetic Nanoparticles. Francis LD, editor. J Nanomater. 2012;2012:454759. doi:10.1155/2012/454759
Abakumov MA, Semkina AS, Skorikov AS, Vishnevskiy DA, Ivanova A V., Mironova E, et al. Toxicity of iron oxide nanoparticles: Size and coating effects. J Biochem Mol Toxicol. 2018;32:e22225. doi:10.1002/JBT.22225
Eslamipour F, Hejazi P. Effects of surface modification and activation of magnetic nanoparticles on the formation of amylase immobilization bonds under different ionic strength conditions. J Mol Catal B Enzym. 2015;119:1–11. doi:10.1016/J.MOLCATB.2015.05.006
Jiang F, Li X, Zhu Y, Tang Z. Synthesis and magnetic characterizations of uniform iron oxide nanoparticles. Phys B Condens Matter. 2014;443:1–5. doi:10.1016/J.PHYSB.2014.03.009
Karaagac O, Kockar H, Tanrisever T. Properties of iron oxide nanoparticles synthesized at different temperatures. J Supercond Nov Magn. 2011;24:675–8. doi:10.1007/S10948-010-0932-4/METRICS
Nguyen DT, Park D-W, Kim K-S. Seed-Mediated Synthesis of Iron Oxide and Gold/Iron Oxide Nanoparticles. Jf Nanosci Nanotechnol. 2011. pp. 7214–7217. doi:10.1166/jnn.2011.4824
Aga-Tagieva SE, Omelyanchik AS, Magomedov KE, Motorzhina A V, Orudzhev FF, Rodionova V V, et al. PEGylated Iron-Oxide Nanoparticles: Structural, Magnetic, and Sorption Properties. Nanobiotechnology Reports. 2023;18:886–893. doi:10.1134/S2635167623600633
Corbett JF. Pseudo first-order kinetics. J Chem Educ. 1972;49:663. doi:10.1021/ED049P663
Robati D. Pseudo-second-orders kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J Nanostructure Chem. 2013;3:1–6. doi:10.1186/2193-8865-3-55
Kumar PS, Ramalingam S, Kirupha SD, Murugesan A, Vidhyadevi T, Sivanesan S. Adsorption behavior of nickel(II) onto cashew nut shell: Equilibrium, thermodynamics, kinetics, mechanism and process design. Chem Eng J. 2011;167. doi:10.1016/j.cej.2010.12.010
Alyasi H, Mackey H, McKay G. Adsorption of Methyl Orange from Water Using Chitosan Bead-like Materials. Mol 2023, Vol 28, Page 6561. 2023;28:6561. doi:10.3390/MOLECULES28186561
WeberJr. WJ, Morris JC. Kinetics of Adsorption on Carbon from Solution. J Sanit Eng Div. 1963;89:31–59. doi:10.1061/JSEDAI.0000430
Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40. doi:10.1021/ja02242a004
Freundlich H. Über die Adsorption in Lösungen. Zeitschrift für Phys Chemie. 1907;57U:385–470. doi:10.1515/zpch-1907-5723
Temkin, M.J. Pyzhev V. Recent modifications to Langmuir isotherms. Acta Physiochim Ussr. 1940;12:217–25.
Dubinin MM, Radushkevich LV. Equation of the characteristic curve of activated charcoal. Proc Acad Sci USSR Phys Chem Sect. 1947;55.
Li H, Jin H, Li R, Hua J, Zhang Z, Li R. Magnetic Fe3O4@SiO2 study on adsorption of methyl orange on nanoparticles. Sci Reports 2024 141. 2024;14:1–16. doi:10.1038/s41598-023-50368-x
Fisli A, Winatapura DS, Alfian A. The Surface Functionalization of Fe3O4 Nanoparticles by CTAB as Adsorbent for Methyl Orange Elimination in Water. J Phys Conf Ser. 2018;1091:012002. doi:10.1088/1742-6596/1091/1/012002
Munagapati VS, Yarramuthi V, Kim DS. Methyl orange removal from aqueous solution using goethite, chitosan beads and goethite impregnated with chitosan beads. J Mol Liq. 2017;240:329–339. doi:10.1016/J.MOLLIQ.2017.05.099
Khajeh M, Barkhordar A. Fe3O4/Graphene Oxide Composite for Adsorption of Methylene Blue and Methyl Orange in Water Treatment. J Appl Spectrosc. 2020;87:701–707. doi:10.1007/S10812-020-01057-4
Phi Y, Song G, Li A, Wang J, Wang H, Sun Y, et al. Graphene oxide-chitosan composite aerogel for adsorption of methyl orange and methylene blue: Effect of pH in single and binary systems. Colloids Surfaces A Physicochem Eng Asp. 2022;641:128595. doi:10.1016/J.COLSURFA.2022.128595
Barakat MA, Kumar R, Lima EC, Seliem MK. Facile synthesis of muscovite–supported Fe3O4 nanoparticles as an adsorbent and heterogeneous catalyst for effective removal of methyl orange: Characterisation, modelling, and mechanism. J Taiwan Inst Chem Eng. 2021;119:146–157. doi:10.1016/J.JTICE.2021.01.025
Zhao Y, Chen H, Li J, Chen C. Hierarchical MWCNTs/Fe3O4/PANI magnetic composite as adsorbent for methyl orange removal. J Colloid Interface Sci. 2015;450:189–195. doi:10.1016/J.JCIS.2015.03.015
Wu L, Liu X, Lv G, Zhu R, Tian L, Liu M, et al. Study on the adsorption properties of methyl orange by natural one-dimensional nano-mineral materials with different structures. Sci Rep. 2021 111. 2021;11:1–11. doi:10.1038/s41598-021-90235-1
Pang J, Han Q, Liu W, Shen Z, Wang X, Zhu J. Two basic bismuth nitrates: [Bi6O6(OH)2](NO3)4 · 2H2O with superior photodegradation activity for rhodamine B and [Bi6O5(OH)3](NO3)5 · 3H2O with ultrahigh adsorption capacity for methyl orange. Appl Surf Sci. 2017;422:283–294. doi:10.1016/J.APSUSC.2017.06.022
Siyasukh A, Chimupala Y, Tonanon N. Preparation of magnetic hierarchical porous carbon spheres with graphitic features for high methyl orange adsorption capacity. Carbon N Y. 2018;134:207–221. doi:10.1016/J.CARBON.2018.03.093
DOI: https://doi.org/10.15826/chimtech.2024.11.3.05
Copyright (c) 2024 Liang Tianhui, Sayara Aga-Tagieva, Alexander Omelyanchik, Zhang Xiaozhou, Hong Lu, Katerina Levada, Valeria Rodionova, Kurban Magomedov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







