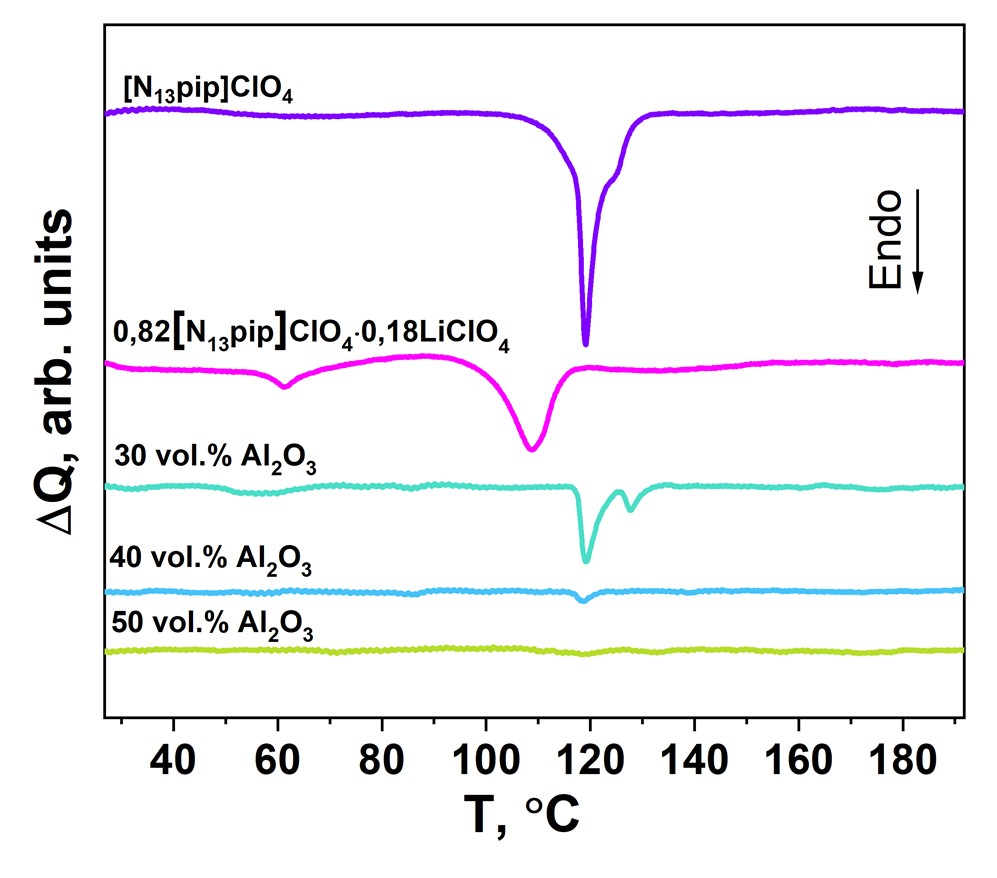
Electrolytes for solid state lithium batteries in the [N13pip]ClO4-LiClO4-Al2O3 system for solid state lithium batteries
Abstract
Keywords
Full Text:
PDFReferences
Li Y, Huang W, Li Y, Pei A, Boyle DT, Cui Y. Correlating Structure and Function of Battery Interphases at Atomic Resolution Using Cryoelectron Microscopy. Joule. 2018;2(10):2167–2177. doi:10.1016/j.joule.2018.08.004
Matsumoto H, Sakaebe H, Tatsumi K. Preparation of Room Temperature Ionic Liquids Based on Aliphatic Onium Cations and Asymmetric Amide Anions and Their Electrochemical Properties as a Lithium Battery Electrolyte. J Power Sources. 2005;146(1):45–50. doi:10.1016/j.jpowsour.2005.03.103
MacFarlane DR, Forsyth M. Plastic Crystal Electrolyte Materials: New Perspectives on Solid State Ionics. Advanced Materials. 2001;13(12–13):957–966. doi:10.1002/1521-4095(200107)13:12/13<957::AID-ADMA957>3.0.CO;2-%23
Timmermans J. Plastic Crystals: A Historical Review. J Phys Chem Solids. 1961;18(1):1–8. doi:10.1016/0022-3697(61)90076-2
Thomas ML, Hatakeyama-Sato K, Nanbu S, Yoshizawa-Fujita M. Organic Ionic Plastic Crystals: Flexible Solid Electrolytes for Lithium Secondary Batteries. Energy Adv. 2023;2(6):748–764. doi:10.1039/D3YA00078H
Macfarlane DR, Kar M, Pringle J. Fundamentals of Ionic Liquids, From Chemistry to Applications. Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim Germany, 2017. doi:10.1002/9783527340033
Kurtoğlu-Öztulum SF, Jalal A, Uzun A. Thermal Stability Limits of Imidazolium, Piperidinium, Pyridinium, and Pyrrolidinium Ionic Liquids Immobilized on Metal Oxides. Journal of Molecular Liquids. 2022;363:119804. doi:10.1016/j.molliq.2022.119804
Matuszek K, Piper SL, Brzęczek-Szafran A, Roy B, Saher S, Pringle JM, MacFarlane DR. Unexpected Energy Applications of Ionic Liquids. Adv Mater. 2024;36(23):2313023. doi:10.1002/adma.202313023
Brutti S, Simonetti E, De Francesco M, Sarra A, Paolone A, Palumbo O, Fantini S, Lin R, Falgayrat A, Choi H, Kuenzel M, Passerini S, Appetecchi GB. Ionic Liquid Electrolytes for High-Voltage, Lithium-Ion Batteries. J Power Sources. 2020;479:228791. doi:10.1016/j.jpowsour.2020.228791
Zhu H, MacFarlane DR, Pringle JM, Forsyth M. Organic Ionic Plastic Crystals as Solid-State Electrolytes. Trends Chem. 2019;1(1):126–140. doi:10.1016/j.trechm.2019.01.002
Park H, Park CB, Sung BJ. Simulation Studies on the Dynamic Heterogeneity of Organic Ionic Plastic Crystals. Bull Korean Chem Soc. 2023;44(9):736–749. doi:10.1002/bkcs.12715
Zhu H, Wang X, Vijayaraghava R, Zhou Y, MacFarlane DR, Forsyth M. Structure and Ion Dynamics in Imidazolium-Based Protic Organic Ionic Plastic Crystals. J Phys Chem Lett. 2018;9(14):3904–3909. doi:10.1021/acs.jpclett.8b01500
MacFarlane DR, Meakin P, Sun J, Amini N, Forsyth M. Pyrrolidinium Imides: A New Family of Molten Salts and Conductive Plastic Crystal Phases. J Phys Chem B. 1999;103(20):4164–4170. doi:10.1021/jp984145s
Jumaah FN, Mobarak NN, Hassan NH, Noor SAM, Nasir SNS, Ludin NA, Badri KH, Ahmad A, Ito ERD, Yoshizawa-Fujita M, Su’ait MS. Review of Non-Crystalline and Crystalline Quaternary Ammonium Ions: Classification, Structural and Thermal Insight into Tetraalkylammonium Ions. J Molecular Liquids. 2023;376:121378. doi:10.1016/j.molliq.2023.121378
Chen F, Jin L, de Leeuw SW, Pringle JM, Forsyth M. Atomistic Simulation of Structure and Dynamics of the Plastic Crystal Diethyl(Methyl)(Isobutyl)Phosphonium Hexafluorophosphate. J Chem Phys. 2013;138(24):244503. doi:10.1063/1.4811179
Biernacka K, Al-Masri D, Yunis R, Zhu H, Hollenkamp AF, Pringle JM. Development of New Solid-State Electrolytes Based on a Hexamethylguanidinium Plastic Crystal and Lithium Salts. Electrochimica Acta. 2020;357:136863. doi:10.1016/j.electacta.2020.136863
Ulihin A, Novozhilov D, Uvarov N. Solid Electrolytes in the N-Propyl-N-Methyl-Pyrrolidinium Tetrafluoroborate—Lithium Tetrafluoroborate System. Batteries. 2023;9(3). doi:10.3390/batteries9030167
Zhou Z-B, Matsumoto H. Lithium-Doped, Organic Ionic Plastic Crystal Electrolytes Exhibiting High Ambient-Temperature Conductivities. Electrochem Commun. 2007;9(5):1017–1022. doi:10.1016/j.elecom.2006.12.012
Seeber A, Forsyth M, M. Forsyth C, A. Forsyth S, Annat G, R. MacFarlane D. Conductivity, NMR and Crystallographic Study of N,N,N,N-Tetramethylammonium Dicyanamide Plastic Crystal Phases: An Archetypal Ambient Temperature Plastic Electrolyte Material. Phys Chem Chem Phys. 2003;5(12):2692–2698. doi:10.1039/B212743A
Uvarov NF. Composite Solid Electrolytes: Recent Advances and Design Strategies. J Solid State Electrochem. 2011;15(2):367–389. doi:10.1007/s10008-008-0739-4
Shekibi Y, Gray-Weale A, MacFarlane DR, Hill AJ, Forsyth M. Nanoparticle Enhanced Conductivity in Organic Ionic Plastic Crystals: Space Charge versus Strain Induced Defect Mechanism. J Phys Chem C. 2007;111(30):11463–11468. doi:10.1021/jp071631j
Ulikhin AS, Izmodenova AV, Uvarov NF. Effect of the Nature of a Heterogeneous Dopant on the Transport and Thermodynamic Properties of Composites Based on N-Methyl-n-Butylpiperidinium Tetrafluoroborate. Russ J Electrochem. 2024;60(1):67–72. doi:10.1134/S1023193524010130
Anion Effects on the Properties of OIPC/PVDF Composites. Materials Advances. 2021;2(5):1683–1694. doi:10.1039/d0ma00992j
Lennert A, Wagner K, Yunis R, Pringle JM, Guldi DM, Officer DL. Efficient and Stable Solid-State Dye-Sensitized Solar Cells by the Combination of Phosphonium Organic Ionic Plastic Crystals with Silica. ACS Appl Mater Interfaces. 2018;10(38):32271–32280. doi:10.1021/acsami.8b12334
Ulikhin AS, Uvarov NF, Kovalenko KA, Fedin VP. Ionic Conductivity of Tetra-n-Butylammonium Tetrafluoroborate in the MIL-101(Cr) Metal-Organic Framework. Microporous and Mesoporous Mater. 2022;332:111710. doi:10.1016/j.micromeso.2022.111710
Maier J. Ionic Conduction in Space Charge Regions. Progress in Solid State Chem. 1995;23(3):171–263. doi:10.1016/0079-6786(95)00004-E
García Y, Porcarelli L, Zhu H, Forsyth M, Mecerreyes D, O’Dell LA. Probing Disorder and Dynamics in Composite Electrolytes of an Organic Ionic Plastic Crystal and Lithium Functionalised Acrylic Polymer Nanoparticles. J Magnetic Resonance Open. 2023;14–15:100095. doi:10.1016/j.jmro.2023.100095
Iranipour N, Gunzelmann DJ, Seeber A, Vongsvivut J, Doherty C, Ponzio F, O’Dell LA, Hollenkamp AF, Forsyth M, Howlett PC. Ionic Transport through a Composite Structure of N-Ethyl-N-Methylpyrrolidinium Tetrafluoroborate Organic Ionic Plastic Crystals Reinforced with Polymer Nanofibres. J Mater Chem A. 2015;3(11):6038–6052. doi:10.1039/C4TA07155G
Sakaebe H, Matsumoto H. N-Methyl-N-Propylpiperidinium Bis(Trifluoromethanesulfonyl)Imide (PP13-TFSI) - Novel Electrolyte Base for Li Battery. Electrochem Commun. 2003;5:594–598. doi:10.1016/S1388-2481(03)00137-1
Bushkova OV, Yaroslavtseva TV, Dobrovolskiy YuA. New Lithium Salts in Electrolytes for Lithium-Ion Batteries (Review). Russ J Electrochem. 2017; 53(7):677–669. doi:10.1134/S1023193517070035
Ulihin AS, Uvarov NF. Electrochemical Properties of Composition Solid Electrolytes LiClO4-MgO. Russ J Electrochem. 2009;45(6):707–710. doi:10.1134/S1023193509060135
Ulihin AS, Uvarov NF, Mateyshina YuG, Brezhneva LI, Matvienko AA. Composite Solid Electrolytes LiClO4–Al2O3. Solid State Ionics. 2006;177(26):2787–2790. doi:10.1016/j.ssi.2006.03.018
Ulihin AS, Uvarov NF, Rabadanov KSh, Gafurov MM, Gerasimov KB. Thermal, Structural and Transport Properties of Composite Solid Electrolytes (1-x)(C4H9)4NBF4–xAl2O3. Solid State Ionics. 2022;378:115889. doi:10.1016/j.ssi.2022.115889
Mateyshina Y, Stebnitskii I, Shivtsov D, Ilyina E, Ulihin A, Bukhtiyarov A, Uvarov N. Hybrid Nanocomposite Solid Electrolytes (n-C4H9)4NBF4–MgO. Int J Molecular Sci. 2023;24(13). doi:10.3390/ijms241310949
Ulihin AS, Uvarov NF. Ionic Conductivity of Composite Solid Electrolytes (C4H9)4NBF4–Al2O3. Russian Journal of Electrochemistry. 2021;57(10):1015–1018. doi:10.1134/S1023193521080140
Wang X, Kerr R, Chen F, Goujon N, Pringle JM, Mecerreyes D, Forsyth M, Howlett PC. Toward High-Energy-Density Lithium Metal Batteries: Opportunities and Challenges for Solid Organic Electrolytes. Advanced Materials. 2020;32(18):1905219. doi:10.1002/adma.201905219
García Y, O’Dell LA. Understanding the Interfacial Region in Organic Ionic Plastic Crystal Composite Electrolyte Materials by Solid-State NMR. Current Opinion in Colloid & Interface Sci. 2022;61:101632. doi:10.1016/j.cocis.2022.101632
Zhu H. Structure and Ion Transport Properties of Organic Ionic Compounds Revealed by NMR. Magnetic Resonance Lett. 2024;4(2):100092. doi:10.1016/j.mrl.2023.11.004
Roggero A, Caussé N, Pébère N, Dantras E. VFT to Arrhenius Crossover at the Dynamic Glass Transition of an Epoxy Network as Revealed by Dielectric Experiments in Continuous Immersion. Polymer. 2022;241:124542. doi:10.1016/j.polymer.2022.124542
Liu GD, Wu JP, Dong HM, Zhang HQ. Nonlinear Modification of Vogel-Fulcher-Tamman (VFT) Model and Its Application in Enthalpy Relaxation of Glassy Polystyrene. J Non-Crystalline Solids. 2020;528:119761. doi:10.1016/j.jnoncrysol.2019.119761
Garca-Coln LS, del Castillo LF, Goldstein P. Theoretical Basis for the Vogel-Fulcher-Tammann Equation. Phys Rev B. 1989;40(10):7040–7044. doi:10.1103/PhysRevB.40.7040
Croce F, Appetecchi GB, Persi L, Scrosati B. Nanocomposite Polymer Electrolytes for Lithium Batteries. Nature. 1998;394(6692):456–458. doi:10.1038/28818
Duan T, Cheng H, Sun Q, Liu Y, Nie W, Chu Y, Xu Q, Lu X. Reinforcing Interfacial Compatibility of LLZTO/PVDF-HFP Composite Electrolytes by Chemical Interaction for Solid-State Lithium Metal Batteries. J Power Sources. 2024;589:233789. doi:10.1016/j.jpowsour.2023.233789
Rahman MYA, Ahmad A, Ismail LHC, Salleh MM. Fabrication and Characterization of a Solid Polymeric Electrolyte of PAN-TiO2-LiClO4. J Appl Polymer Sci. 2010;115(4):2144–2148. doi:10.1002/app.31299
DOI: https://doi.org/10.15826/chimtech.2024.11.3.04
Copyright (c) 2024 Daria Kyzlasova, Artem Ulihin, Nikolai Uvarov

This work is licensed under a Creative Commons Attribution 4.0 International License.
© Website Chimica Techno Acta, 2014–2024
ISSN 2411-1414 (Online)
This journal is licensed under a Creative Commons Attribution 4.0 International







