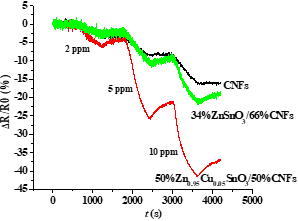
Synthesis of copper-doped nanocrystalline tin stannate by thermal decomposition of a precursor
Abstract
Keywords
Full Text:
PDFReferences
Li X, Guan G, Yu C, Cheng B, Chen X, Zhang K, Xiang J. Enhanced electrochemical performances based on ZnSnO3 microcubes functionalized in-doped carbon nanofibers as free-standing anode materials. Dalton Trans. 2023;52(32):11187–11195. doi:10.1039/D3DT01642K
Zhang Y, Wei J-L, Jin X-Y, Yu M-C, Wang L, Guo Yu-H, Dong S-T. Electrospun ZnSnO3/C Nanofibers as an Anode Material for Lithium-Ion Batteries. J Electron Mater. 2021;50:4945–4953. doi:10.1007/s11664-021-09036-x
Ma Y, Jiang R, Li D, Dong Y, Liu Y, Zhang J. Embedding ultrafine ZnSnO3 nanoparticles into reduced graphene oxide composites as high-performance electrodes for lithium ion batteries. Nanotechnol. 2018;29(19):195401. doi:10.1088/1361-6528/aab07e
Wang D, Pu X, Yu X, Bao L, Cheng Y, Xu J, Han S, Ma Q, Wang X. Controlled preparation and gas sensitive properties of two-dimensional and cubic structure ZnSnO3. J Colloid Interface Sci. 2022 608(1):1074–1085. doi:10.1016/j.jcis.2021.09.167
Wei JL, Jin XY, Yu MC, Wang L, Guo YH, Dong ST, Zhang YM. Electrospun ZnSnO3/C nanofibers as an anode material for lithium-ion batteries. J Electron Mater. 2021;50:4945–4953. doi:10.1007/s11664-021-09036-x
Wang K, Zhang S, Hou Z, Wang L, An P, Jia J, Li Y, Zhang P. Nanofibrous ZnSnO3/C composite derived from natural cellulose substance as an enhanced lithium-ion battery anode. Mater. Lett. 2023;331:133435. doi:10.1016/j.matlet.2022.133435
Sim CK, Majid SR, Mahmood NZ. Synthesis of highly porous carbon/ZnSnO3 composite and its electrochemical properties. J Energy Storage. 2020;32:101843. doi:10.1016/j.est.2020.101843
Sim CK, Majid SR, Mahmood NZ. ZnSnO3/ mesoporous biocarbon composite towards sustainable electrode material for energy storage device. Microchem J. 2021;164:105968. doi:10.1016/j.microc.2021.105968
Rahman M, Bashar MS, Rahman Md. L, Chowdhury FI. Comprehensive review of micro/nanostructured ZnSnO3: characteristics, synthesis, and diverse applications. RSC Advances. 2023;13(44):30798–30837. doi:10.1039/ d3ra05481k
Ma X, Dong X, Li B, Zheng Q, Li R, Huang C, Huo L, Zhang X, Cheng X, Xu Y. Construction of amorphous ZnSnO3 micro/nanostructure material for low concentration n-pentanol detection. Ceram Int. 2024;50:25122–25130. doi:10.1016/j.ceramint.2024.04.241
Sun B, Sima Z, Wang Q, Song P. Effect of in doping on the formaldehyde sensing performance of ZnSnO3 cubes ceramics. Ceram Int. 2023;49:39588–39596. doi:10.1016/j.ceramint.2023.09.310
Jiang L, Xue K, Chen Z, Cui Q, Xu S. High performance of gas sensor based on Bi-doped ZnSnO3/CuO nanocomposites for acetone. Microporous Mesoporous Mater. 2022;329:111532. doi:10.1016/j.micromeso.2021.111532
Yu S, Jia X, Yang J, Wang S, Li Y, Song H. Highly sensitive ethanol gas sensor based on CuO/ZnSnO3 heterojunction composites. Mater Lett. 2021;291:129531. doi:10.1016/j.matlet.2021.129531
Sun C, Shao J, Wang Z, Liu H, Li Z, Zhang H, Bai T, Sun Y, Guo L, Pan G, Yang X. CuO-sensitized amorphous ZnSnO3 hollow-rounded cubes for highly sensitive and selective H2S gas sensors. Sens Actuators B Chem. 2022;362:131799. doi:10.1016/j.snb.2022.131799
Gao S, Wang C , Li X, Yuan R, Zhang Q, Zhao J, Chu H. Amorphous CoSnO3 for conductometric triethylamine gas sensing. Sens Actuators B Chem. 2024;401:135086. doi:10.1016/j.snb.2023.135086
Yan S, Zhang S-Z, Xie W-F, Gai L-Y, Yuan H-M, Zhang D, Zhang H, Liu X, Yang W, Chi Z-T. Chemiresistive ethanol sensors based on In2O3/ZnSnO3 nanocubes. Sens Actuators Rep. 2022;4:100099. doi:10.1016/j.snr.2022.100099
Wang XY, Wang MM, Xin JH, Yang Z, Leng BX. The enhanced practicability of ZnSnO3 sensors based on Co, Ni co-doping and N719 dye sensitization. Sens Actuators B Chem. 2022;362:131787. doi:10.1016/j.snb.2022.131787
Zheng J, Hou H, Fu H, Gao L, Liu H. Size-controlled synthesis of porous ZnSnO3 nanocubes for improving formaldehyde gas sensitivity. RSC Adv. 2021;11:20268–20277. doi:10.1039/d1ra01852c
Liu K, Zheng Z, Xu J, Zhang C. Enhanced visible light-excited ZnSnO3 for room temperature ppm-level CO2 detection. J Alloys Compd. 2022;907:164440. doi:10.1016/j.jallcom.2022.164440
Ochoa-Muñoz YH, Rodríguez-Páez JE, Mejía de Gutiérrez R. Structural and optical study of perovskite nanoparticles MSnO3 (M = Ba, Zn, Ca) obtained by a wet chemical route. Mater Chem Phys. 2021;266:124557. doi:10.1016/j.matchemphys.2021.124557
Martínez-Aguilar E, Hmŏk H'L, Siqueiros JM, López-Juárez R. Tuning of the ferroelectric and optical properties of ZnSnO3 with transition metals: Mn, Cr and Co. Mater Chem Phys. 2024;319:129387. doi:10.1016/j.matchemphys.2024.129387
Li D, Yan P, Zhao Q, Wang L, Ma X, Xue J, Zhang Y, Liu M. The hydrothermally synthesis of ZnSn(OH)6 and Zn2SnO4 and their photocatalytic performances. Cryst Eng Comm. 2020;20:4923–4932. doi:10.1039/d0ce00777c
Wu Z, Fan B, Zhang L, Yao Y, Hong S, Yu H, Jia Y. Strongly enhanced piezoelectric-catalysis of ZnSnO3 /graphite hybrid materials for dye wastewater decomposition. Ceram Int. 2023;49(18):29614–29621. doi:10.1016/j.ceramint.2023.06.180
Najorka J, Kleppe AK, Welch MD. The effect of pressure and composition on Cu-bearing hydroxide perovskite. Phys Chem Minerals. 2019;46:877–887. doi:10.1007/s00269-019-01047-9
Anucha CB, Altin I, Bacaksiz E, Stathopoulos VN, Polat I, Yasar A, Yüksel ÖF. Silver doped zinc stannate (Ag-ZnSnO3) for the photocatalytic degradation of caffeine under UV irradiation. Water. 2021;13:1290. doi:10.3390/w13091290
Soltani A, Djani F, Mazouzi DE, Tiri RNE, Aygün A, Şen F, Martinez-Arias A. ZnSnO3-SnO2 nanocomposite as a catalyst for efficient hydrogen production through sodium borohydride methanolysis. Int J Hydrog Energy. 2024;67:429–437. doi:10.1016/j.ijhydene.2024.04.208
Mayedwa N, Mongwaketsi N, Khamlich S, Kaviyarasu K, Matinise N, Maaza M. Green synthesis of zin tin oxide (ZnSnO3) nanoparticles using Aspalathus Linearis natural extracts: structural, morphological, optical and electrochemistry study. Appl Surf Sci. 2018;446:250–257. doi:10.1016/j.apsusc.2017.12.161
Loginov AV, Mateyshina YG, Aparnev AI, Uvarov NF. Synthesis of BaSnO3/SnO2 nanocomposites as heterogeneous additive for composite solid electrolytes. Russ J Appl Chem. 2018;91(10):1660–1664. doi:10.1134/S1070427218100130
Loginov AV, Aparnev AI, Uvarov NF. Synthesis of SrSnO3/SnO2 composites via thermal decomposition of a precursor. Inorg Mater. 2022;58(8):420–424. doi:10.1134/S0020168522040094
Loginov AV, Aparnev AI, Uvarov NF. Nanocomposites Prepared via Thermal Decomposition of Calcium Hydroxystannate CaSn(OH)6. Inorg Mater. 2022;58(8):814–821. doi:10.1134/S0020168522080088
Dong S, Cui L, Zhao Y, Wu Y, Xia L, Su X, Zhang C, Wang D, Guo W, Sun J. Crystal structure and photocatalytic properties of perovskite MSn(OH)6 (M = Cu and Zn) composites with d10-d10 configuration. Appl Surf Sci. 2019;463:659–667. doi:10.1016/j.apsusc.2018.09.006
Marshukova NK, Palovskii AB, Sidorenko GA, Chistyakova NI. Vismirnovite, ZnSn(OH)6, and natanite, FeSn(OH)6, new tin minerals. Zap Vser Mineral Obshch. 1981;110:492–500.
Yasser H, Rodriguez-Paez JE, Gutierrez RM. Structural and optical study of perovskite nanoparticles MSnO3 (M = Ba, Zn, Ca) obtained by a wet chemical route. Mater Chem Phys. 2021;266:124557. doi:10.1016/j.matchemphys.2021.124557
Bannov AG, Lapekin NI, Kurmashov PB, Ukhina AV, Manakhov A. Room-Temperature NO2 Gas Sensors Based on Granulated Carbon Nanofiber Material. Chemosensors. 2022;10(12):525. doi:10.3390/chemosensors10120525
Kuvshinov GG, Mogilnykh YI, Kuvshinov DG. Kinetics of carbon formation from CH4-H2 mixtures over a nickel containing catalyst. Catal Today. 2021;42:357–360. doi:10.1016/S0920-5861(98)00115-1
Sivapunniyam A, Wiromrat N, Myint MTZ, Dutta J. High-performance liquefied petroleum gas sensing based on nanostructures of zinc oxide and zinc stannate. Sensors Actuators B Chem. 2011;157:232–239. doi:10.1016/j.snb.2011.03.055
Ganesan M, Jayaraman V, Selvaraj P, Mani KM, Kim DH. Pyrochlore cerium stannate (Ce2Sn2O7) for highly sensitive NO2 gas sensing at room temperature. Appl Surf Sci. 2023;624:157135. doi:10.1016/j.apsusc.2023.157135
DOI: https://doi.org/10.15826/chimtech.2024.11.4.05
Copyright (c) 2024 Alexander Aparnev, Anton Loginov, Alexander Bannov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







