Layered and hexagonal perovskites as novel classes of proton-conducting solid electrolytes. A focus review
Abstract
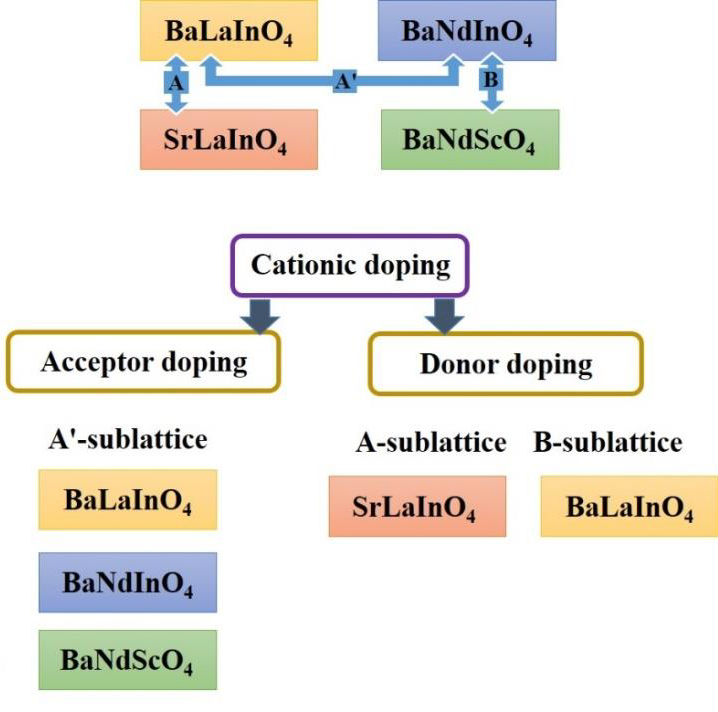
Solid oxide electrolytes have attracted significant attention due to their possible applications in energy conversion devices, including solid oxide fuel cells (SOFCs) and electrolysis cells (SOECs). Although a large amount of data has been accumulated to date, the design of new representatives of ionic electrolytes is of unquenchable interest. In this paper, a review of the new classes of proton-conducting solid electrolytes is provided. The physicochemical and transport properties of layered perovskites (BaNdInO4, BaNdScO4, SrLaInO4, BaLaInO4) and hexagonal perovskites (Ba7Nb4MoO20, Ba5Er2Al2ZrO13 and Ba5In2Al2ZrO13) were analyzed and summarized. Based on the performed analysis, the most promising compositions among the considered phases were identified and the effective approaches aimed at improving their functional characteristics were provided.
Keywords
Full Text:
PDFReferences
Filippov SP, Yaroslavtsev AB, Hydrogen energy: development prospects and materials, Russ. Chem. Rev. 90(6) (2021) 627-643. https://doi.org/10.1070/RCR5014
Istomin SYa, Lyskov NV, Mazo GN, Antipov EV, Electrode materials based on complex d-metal oxides for symmetrical solid oxide fuel cells, Russ. Chem. Rev. 90(6) (2021) 644-676. https://doi.org/10.1070/RCR4979
Nikonov AV, Pavzderin NB, Khrustov VR, Properties of the La0.6Sr0.4Co0.8Fe0.2O3–δ – Ce0.73Gd0.27O2–δ Composite Cathode Formed from Nanopowders, Russ. J. Electrochem. 58 (2022) 311-320. https://doi.org/10.1134/S1023193522040103
Voloshin BV, Koshevoi EI, Ulihin AS, Popov MP, et al., Modifying the La0.6Sr0.4Co0.2Fe0.8O3–δ Cathodic Material by Ferroactive Molybdenum Cation, Russ. J. Electrochem. 58 (2022) 163-167. https://doi.org/10.1134/S1023193522020112
Nefedkin SI, Ivanenko AV, Pavlov VI, Panov SV, et al., Hydrogen–Air Fuel Cells with Open Cathode for High-Rate Electric Energy Systems, Russ. J. Electrochem. 58 (2022) 151-162. https://doi.org/10.1134/S1023193522020082
Ivanov AI, Bredikhin SI, Kharton VV, Mixed Ionic-Electronic Conductivity of the Fluorite-Type Ce1–x–yLaxPryO2–δ Solid Solutions under Reducing Conditions, Russ. J. Electrochem. 58 (2022) 122-130. https://doi.org/10.1134/S1023193522020045
Agarkova EA, Zadorozhnaya OYu, Burmistrov IN, Agarkov DA, et al., Tape Casting of Bilayered Anode Supports and Electrochemical Performance of SOFCs Based on Them, Russ. J. Electrochem. 58 (2022) 114-121. https://doi.org/10.1134/S1023193522020033
Voloshin BV, Bulina NV, Popov MP, Nemudry AP, Operando X-Ray Diffraction Analysis of a Microtubular La0.6Sr0.4Co0.2Fe0.8O3–δ Membrane, Russ. J. Electrochem. 58 (2022) 100-104. https://doi.org/10.1134/S1023193522020100
Lyskov NV, Galin MZ, Napol’skii PhS, Mazo GN, Increasing the Electrochemical Activity of the Pr1.95La0.05CuO¬4 Cathode by Laser Modification of the Electrode/Electrolyte Interface Profile, Russ. J. Electrochem. 58 (2022) 93-99. https://doi.org/10.1134/S1023193522020070
Kasyanova AV, Tarutina LR, Rudenko AO, Laygaeva JG, et al., Ba(Ce,Zr)O3-based electrodes for protonic ceramic electrochemical cells: towards highly compatible functionality and triple-conducting behavior, Russ. Chem. Rev. 86(6) (2020) 667-692. https://doi.org/10.1070/RCR4928
Lomonova EE, Agarkov DA, Borik MA, Korableva GM, et al., Structure and Transport Characteristics of Single-Crystal and Ceramic ZrO2–Y2O3 Solid Electrolytes, Russ. J. Electrochem. 58 (2022) 105-113. https://doi.org/10.1134/S1023193522020069
Kunshina GB, Bocharova IV, Shcherbina OB, Electrical Conductivity and Mechanical Properties of Li7-3xAlxLa3Zr2O12 Solid Electrolyte, Inorg. Mater. 58 (2022) 147-153. https://doi.org/10.1134/S0020168522020091
Ruddlesden SN, Popper P, New compounds of the K2NiF4 type, Acta Crystallogr. 10 (1957) 538-539. https://doi.org/10.1107/S0365110X57001929
Page YLe, Siegrist T, Sunshine SA, Schneemeyer LF, et al., Structural properties of Ba2RCu3O7 high-Tc superconductors, Phys. Rev. B. 36 (1987) 3517-3621. https://doi.org/10.1103/PhysRevB.36.3617
Cheong S-W, Thompson JD, Fisk Z, Properties of La2CuO4 and related compounds, Physica C. 158 (1989) 109-126. https://doi.org/10.1016/0921-4534(89)90306-7
Tokura Y, Takagi H, Uchida S, A superconducting copper oxide compound with electrons as the charge carriers, Nature. 337 (1989) 345-347. https://doi.org/10.1038/337345a0
Hauck J, Mika K, Krabbes G, Search for new high Tc oxide structures, Physica C. 185-189 (1991) 721-722. https://doi.org/10.1016/0921-4534(91)92163-6
Gangily P, Rao NR, Crystal Chemistry and Magnetic Properties of Layered Metal Oxides Possessing the K2NiF4 or Related Structures, J. Solid State Chem. 53 (1984) 193-216. https://doi.org/10.1016/0022-4596(84)90094-X
Kawano S, Achiwa N, Kamegashira N, Aoki M, Magnetic properties of K2NiF4 type oxides, SrLaMnO4+x (0 ≤ x ≤ 0.2), J. Phys. Colloques. 49 (1988) 829-830. https://doi.org/10.1051/jphyscol:19888373
Moritomo Y, Tomioka Y, Asamitsu A, Tokura Y, Magnetic and electronic properties in hole-doped manganese oxides with layered structures: La1−xSr1+xMnO4, Phys. Rev. B 51 (1995)3297-3300. https://doi.org/10.1103/PhysRevB.51.3297
Hector AL, Knee CS, MacDonald AI, Price DJ, et al., An unusual magnetic structure in Sr2FeO3F and magnetic structures of K2NiF4-type iron (III) oxides and oxide halides, including the cobalt substituted series Sr2Fe1−xCoxO3Cl, J. Mater. Chem. 15 (2005) 3093–3103. https://doi.org/10.1039/B505617A
Amow G, Davidson IJ, Skinner SJ, A comparative study of the Ruddlesden-Popper series, Lan+1NinO3n+1 (n=1, 2 and 3), for solid-oxide fuel-cell cathode applications, Solid State Ion. 177 (2006) 1205–1210. https://doi.org/10.1016/j.ssi.2006.05.005
Amow G, Skinner SJ, Recent developments in Ruddlesden–Popper nickelate systems for solid oxide fuel cell cathodes, J. Solid State Electrochem. 10 (2006) 538–546. https://doi.org/10.1007/s10008-006-0127-x
Sayers R, Liu J, Rustumji B, Skinner SJ, Novel K2NiF4-Type Materials for Solid Oxide Fuel Cells: Compatibility with Electrolytes in the Intermediate Temperature Range, Fuel Cell. 08 (2008) 338–343. https://doi.org/10.1002/fuce.200800023
Takahashi S, Nishimoto S, Matsuda M, Miyake M, Electrode Properties of the Ruddlesden–Popper Series, Lan+1NinO3n+1 (n = 1, 2 and 3), as Intermediate-Temperature Solid Oxide Fuel Cells, J. Am. Ceram. Soc. 93 (2010) 2329–2333. https://doi.org/10.1111/j.1551-2916.2010.03743.x
Montenegro-Hernandez A, Vega-Castillo J, Mogni L, Caneiro A, Thermal stability of Ln2NiO4+δ (Ln: La, Pr, Nd) and their chemical compatibility with YSZ and CGO solid electrolytes, Int. J. Hydrog. Energy. 36(24) (2011) 15704-15714. https://doi.org/10.1016/j.ijhydene.2011.08.105
Grimaud A, Mauvy F, Bassat JM, Fourcade S, et al., Hydration and transport properties of the Pr2-xSrxNiO4+δ compounds as H+-SOFC cathodes, J. Mater. Chem. 22 (2012) 16017-16025. https://doi.org/10.1039/C2JM31812A
Ferkhi M, Ahmed Yahia H, Electrochemical and morphological characterizations of La2-xNiO4+δ (x = 0.01, 0.02, 0.03 and 0.05) as new cathodes materials for IT-SOFC, Mater. Res. Bull. 83 (2016) 268–274. https://doi.org/10.1016/j.materresbull.2016.06.009
Sharma RK, Burriel M, Dessemond L, Bassat JM, et al., Lan+1NinO3n+1 (n = 2 and 3) phases and composites for solid oxide fuel cell cathodes: Facile synthesis and electrochemical properties, J. Power Sources. 325 (2016) 337-345. https://doi.org/10.1016/j.jpowsour.2016.06.047
Vibhu V, Rougier A, Nicollet C, Flura A, et al., Pr4Ni3O10+δ: A new promising oxygen electrode material for solid oxide fuel cells, J. Power Sources. 317 (2016) 184-193. https://doi.org/10.1016/j.jpowsour.2016.03.012
Yatoo MA, Du Z, Zhao H, Aguadero A, et al., La2Pr2Ni3O10±δ Ruddlesden-Popper phase as potential intermediate temperature-solid oxide fuel cell cathodes, Solid State Ion. 320 (2018) 148–151. https://doi.org/10.1016/j.ssi.2018.02.043
Morales-Zapata MA, Larrea A, Laguna-Bercero MA, Reversible operation performance of microtubular solid oxide cells with a nickelate-based oxygen electrode, Int. J. Hydrog. Energy. 45(8) (2020) 5535-5542. https://doi.org/10.1016/j.ijhydene.2019.05.122
Danielson E, Devenney M, Giaquinta DM, Golden JH, et al., X-ray powder structure of Sr2CeO4: A new luminescent material discovered by combinatorial chemistry, J. Mol. Struct. 470 (1998) 229-235. https://doi.org/10.1016/S0022-2860(98)00485-2
Li J, Li X, Hu S, Li Y, et al., Photoluminescence mechanisms of color-tunable Sr2CeO4: Eu3+, Dy3+ phosphors based on experimental and first-principles investigation, Opt. Mater. 35(12) (2013) 2309-2313. https://doi.org/10.1016/j.optmat.2013.06.024
Sahu M, Gupta SK, Jain D, Saxena MK, et al., Solid state speciation of uranium and its local structure in Sr2CeO4 using photoluminescence spectroscopy, Spectrochim. Acta A. 195 (2018) 113-119. https://doi.org/10.1016/j.saa.2018.01.048
Jia Y, Shen S, Wang D, Wang X, et al., Composite Sr2TiO4/SrTiO3(La,Cr) heterojunction based photocatalyst for hydrogen production under visible light irradiation, J. Mater. Chem. A. 1 (2013) 7905–7912. https://doi.org/10.1039/c3ta11326d
Zhang H, Ni S, Mi Y, Xu X, Ruddlesden-Popper compound Sr2TiO4 co-doped with La and Fe for efficient photocatalytic hydrogen production, J. Catal. 359 (2018) 112-121. https://doi.org/10.1016/j.jcat.2017.12.031
Ziati M, Bekkioui N, Ez-Zahraouy H, Ruddlesden-Popper compound Sr2TiO4 doped with chalcogens for optoelectronic applications: Insights from first-principle calculations, J. Chem. Phys. 548 (2021) 111221. https://doi.org/10.1016/j.chemphys.2021.111221
Tarasova N, Animitsa I, Protonic transport in oxyfluorides Ba2InO3F and Ba3In2O5F2 with Ruddlesden-Popper structure, Solid State Ion. 275 (2015) 53–57. https://doi.org/10.1016/j.ssi.2015.03.025
Zhou Y, Shiraiwa M, Nagao M, Fujii K, et al., Protonic Conduction in the BaNdInO4 Structure Achieved by Acceptor Doping, Chem. Mater. 33 (2021) 2139 - 2146. https://doi.org/10.1021/acs.chemmater.0c04828
Shiraiwa M, Kido T, Fujii K, Yashima M, High-temperature proton conductors based on the (110) layered perovskite BaNdScO4, J. Mat. Chem. A. 9 (2021) 8607. https://doi.org/10.1039/D0TA11573H
Troncoso L, Arce MD, Fernandez-Diaz MT, Mogni LV, et al., Water insertion and combined interstitial-vacancy oxygen conduction in the layered perovskites La1.2Sr0.8-xBaxInO4+d, New J. Chem. 43 (2019) 6087. https://doi.org/10.1039/C8NJ05320K
Tarasova N, Animitsa I, Galisheva A, Korona D, Incorporation and Conduction of Protons in Ca, Sr, Ba-Doped BaLaInO4 with Ruddlesden-Popper Structure, Materials. 12 (2019) 1668. https://doi.org/10.3390/ma12101668
Tarasova N, Animitsa I, Galisheva A, Pryakhina V, Protonic transport in the new phases BaLaIn0.9M0.1O4.05 (M=Ti, Zr) with Ruddlesden-Popper structure, Solid State Sci. 101 (2020) 106121. https://doi.org/10.1016/j.solidstatesciences.2020.106121
Tarasova N, Animitsa I, Galisheva A, Electrical properties of new protonic conductors Ba1+хLa1–хInO4–0.5х with Ruddlesden-Popper structure, J. Solid State Electrochem. 24 (2020) 1497-1508. https://doi.org/10.1007/s10008-020-04630-1
Tarasova N, Galisheva A, Animitsa I, Improvement of oxygen-ionic and protonic conductivity of BaLaInO4 through Ti doping, Ionics. 26 (2020) 5075-5088. https://doi.org/10.1007/s11581-020-03659-6
Tarasova N, Galisheva A, Animitsa I, Ba2+/Ti4+- co-doped layered perovskite BаLaInO4: the structure and ionic (O2−, H+) conductivity, Int. J. Hydrog. Energy 46(32) (2021) 16868-16877. https://doi.org/10.1016/j.ijhydene.2021.02.044
Tarasova N, Galisheva A, Animitsa I, Korona D, Hydration and the State of Oxygen–Hydrogen Groups in the Complex Oxide BaLaIn0.9Nb0.1O4.1 with the Ruddlesden–Popper Structure, Russian J. Phys. Chem. A. 94 (2020) 818-821. https://doi.org/10.1134/S0036024420030309
Tarasova N, Galisheva A, Animitsa I, Dmitrieva A, The Effect of Donor Doping on the Ionic (О2–, Н+) Transport in Novel Complex Oxides BaLaIn1– xNbxO4+x with the Ruddlesden–Popper Structure, Russian J. Electrochem. 57 (2021) 962-969. https://doi.org/10.1134/S1023193521080115
Tarasova N, Animitsa I, Galisheva A Effect of acceptor and donor doping on the state of protons in block-layered structures based on BaLaInO4, Solid State Commun. 323 (2021) 14093. https://doi.org/10.1016/j.ssc.2020.114093
Tarasova N, Animitsa I, Galisheva A, Effect of doping on the local structure of new block-layered proton conductors based on BaLaInO4, J. Raman Spectrosc. 51 (2020) 2290-2297. https://doi.org/10.1002/jrs.5966
Tarasova N, Animitsa I, Galisheva A, Spectroscopic and transport properties of Ba- and Ti-doped BaLaInO4, J. Raman Spectrosc. 52 (2021) 980-987. https://doi.org/10.1002/jrs.6078
Kreuer K, Proton-Conducting Oxides, Ann. Rev. Mater. Res. 33 (2003) 333-359. https://doi.org/10.1146/annurev.matsci.33.022802.091825
Duan C, Huang J, Sullivan N, O'Hayre R. Proton-conducting oxides for energy conversion and storage, Applied Physics Reviews. 7 (2020) 011314. https://doi.org/10.1063/1.5135319
Fop S, et al., Oxide ion conductivity in the hexagonal perovskite derivative Ba3MoNbO8.5, J. Am. Chem. Soc. 138 (2016) 16764–16769. https://doi.org/10.1021/jacs.6b10730
Katz L, Ward R, Structure relations in mixed metal oxides, Inorg. Chem. 3 (1964) 205–211. https://doi.org/10.1021/ic50012a013
Darriet J, Subramanian MA, Structural relationships between compounds based on the stacking of mixed layers related to hexagonal perovskite-type structures, J. Mater. Chem. 5 (1995) 543-552. https://doi.org/10.1039/JM9950500543
Garcıa-Gonzalez E, Parras M, Gonzalez-Calbet JM, Crystal Structure of an Unusual Polytype:7H-Ba7Nb4MoO20, Chem. Mater. 11 (1999) 433-437. https://doi.org/10.1021/cm981011i
Fop S, McCombie KS, Wildman EJ, Skakle JMS, et al., High oxide ion and proton conductivity in a disordered hexagonal perovskite, Nature Mater. 19 (2020) 752–757. https://doi.org/10.1038/s41563-020-0629-4
Yashima M, Tsujiguchi T, Sakuda Y, Yasui Y, et al., High oxide-ion conductivity through the interstitial oxygen site in Ba7Nb4MoO20-based hexagonal perovskite related oxides, Nature Comm. 12 (2021) 556. https://doi.org/10.1038/s41467-020-20859-w
Abakumov AM, Antipov EV, Kovba LM, Kopnin EM, et al., Complex oxides with coherent intergrowth structures, Russ. Chem. Rev. 64(8) (1995) 719-729. https://doi.org/10.1070/RC1995v064n08ABEH000171
Murakami T, Hester JR, Yashima M, High Proton Conductivity in Ba5Er2Al2ZrO13, a Hexagonal Perovskite-Related Oxide with Intrinsically Oxygen-Deficient Layers, J. Am. Chem. Soc. 142 (2020) 11653–11657. https://doi.org/10.1021/jacs.0c02403
Shpanchenko R, Abakumov A, Antipov E, Kovba L, Crystal structure of Ba5In2Al2ZrO13, J. Alloy. Compd. 206 (1994) 185–188. https://doi.org/10.1016/0925-8388(94)90033-7
Andreev R, Korona D, Anokhina I, Animitsa I. Proton and Oxygen-Ion Conductivities of Hexagonal Perovskite Ba5In2Al2ZrO13, Materials. 15 (2022) 3944. https://doi.org/10.3390/ma15113944
DOI: https://doi.org/10.15826/elmattech.2022.1.004
Copyright (c) 2022 Natalia A. Tarasova, Irina E. Animitsa, Anzhelika O. Galisheva, Dmitry A. Medvedev

This work is licensed under a Creative Commons Attribution 4.0 International License.

