Wetting and spreading phenomena in liquid bismuth-alkali halide systems
Abstract
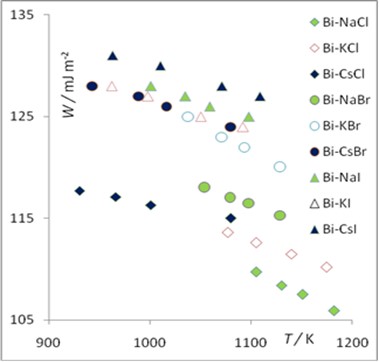
This investigation is devoted to the study of the features of wetting phenomena in high temperature systems that are promising as environments for processing and disposal of man-made and radioactive wastes. New data on the phase wetting transition in two-phase liquid media have been obtained by analyzing the values of the adhesion of salt melts to liquid metals. The present study is focused on the systems composed of molten alkali halides and liquid bismuth. Their adhesion was calculated using experimental data on the surface tension of metals and salts, as well as the interfacial tension between metals and salts. A regular change in the adhesion work is illustrated depending on the temperature, electrical potential and nature of the contact phases. The transition phenomenon from incomplete wetting to full wetting has been established. It is shown that this transition is facilitated by an increase in temperature, a potential jump in the phase contact plane, and the polarizability of salt phase ions. The potential dependence of the adhesion work near the wetting transition point is described by a power equation with a critical index equal to 1.1.
Keywords
Full Text:
PDFReferences
Baymakov YuV, Vetyukov MM. Elektroliz rasplavlennykh soley [Electrolysis of Molten Salts]. Metallurgy: Moscow; 1966. 560 p. Russian.
Suzdaltsev AV, Pershin PS, Filatov AA, Nikolaev AYu, Zaikov YuP, Synthesis of aluminum master alloys in oxide-fluoride melts: A Review, J. Electrochem. Soc. 167 (2020) 102503. https://doi.org/10.1149/1945-7111/ab9879
Brodova IG, Petrova AN, Shirinkina IG, Rasposienko DYu, et al., Mechanical properties of submicrocrystalline aluminium matrix composites reinforced by “in situ” graphene through severe plastic deformation processes, J. Alloys. Comp. 859 (2021) 158387. https://doi.org/10.1016/j.jallcom.2020.158387
Brodova I, Yolshina L, Razorenov S, Rasposienko D, et al., Effect of grain size on the properties of aluminum matrix composites with graphene, Metals, 12 (6) (2022) 1054. https://doi.org/10.3390/met12061054
Barbin NM, Kazantsev GF, Vatolin NA, Pererabotka vtorichnogo svintsovogo syr'ya v rasplavakh ionnykh soley [Processing of Secondary Lead Raw Materials in Ionic Salt Melts]. UrO RAN: Yekaterinburg; 2002. 200 p. Russian.
Arkhipov PA, Zaikov YuP, Khalimullina YuKh, Kholkina AS, Arkhipov SP, Electrochemical behavior of lead, silver and bismuth containing alloys in the KCl-PbCl2 melt, J. Mol. Liquids, 361 (2022) 119619. https://doi.org/10.1016/j.molliq.2022.119619
Lebedev VA, Izbiratel'nost' zhidkometallicheskikh elektrodov v rasplavlennykh galogenidakh [Selectivity of Liquid Metal Electrodes in Molten Halides]. Metallurgy: Chelyabinsk; 1993. 232 p. Russian.
Smolenski V, Novoselova A, Electrochemical separation of uranium from dysprosium in molten salt/liquid metal extraction system, J. Electrochem. Soc. 168 (2021) 062505. https://doi.org/10.1149/1945-7111/ac0557
Mirza M, Abdulaziz R, Maskell W, Wilcock S, et al., Electrochemical processing in molten salts – a nuclear perspective, Energy. Environ. Sci. 16 (2023) 952–982. https://doi.org/10.1039/D2EE02010F
Polyakov PV, Isaeva LA, Mikhalev YuG, Spontaneous surface convection during electrolysis of molten salts with a liquid electrode, (Elektrokhimiya) Electrochemistry, 16 (1980) 1132–1138.
Isaeva LA, Polyakov PV, Mikhalev YuG, Rogozin YuN, New modes of interphase convection during electrolysis of molten salts with a liquid electrode, (Elektrokhimiya) Electrochemistry, 20 (1984) 957–962.
Tanaka T, Nakamoto M, Oguni R, Lee J, Hara S, Measurement of the surface tension of liquid Ga, Bi, Sn, In and Pb by the constrained drop method, Journal of Materials Research, 95 (9) (2004) 818–822. https://doi.org/10.1515/ijmr-2004-0151
Smirnov MV, Stepanov VP, Density and surface tension of molten alkali halides and their binary mixtures. Electrochimica Acta, 27 (11) (1982) 1551–1563. https://doi.org/10.1016/0013-4686(82)80082-0
Smirnov M, Stepanov V, Sharov Interfacial tension of liquid lead in molten alkali chlorides, Dokl. USSR Academy Sci. 197 (1971) 631–634.
Stepanov V, Smirnov M, Interfacial tension and zero charge potentials of liquid lead and bismuth in molten alkali metal halides, Dokl. USSR Academy Sci. 227 (1976) 403–406.
Smirnov M, Stepanov V, Sharov A, Minchenko V, Electrocapillary phenomena on liquid indium and bismuth in molten alkali chlorides, (Elektrokhimiya) Electrochemistry, 8 (1972) 994–999.
Smirnov M, Stepanov V, Sharov A, Electrocapillary phenomena on liquid bismuth in molten mixtures of potassium chloride and bromide, (Elektrokhimiya) Electrochemistry, 12 (1976) 600–602.
Smirnov M, Stepanov V, Sharov A, Electrocapillary phenomena at the boundary of liquid bismuth with binary mixtures of potassium halides. (Elektrokhimiya) Electrochemistry, 12 (1976) 1728–1730.
Smirnov M, Stepanov V, Korkin A, Electrocapillary phenomena on liquid bismuth in molten mixtures NaI–KI, NaI–CsI, (Elektrokhimiya) Electrochemistry, 15 (1979) 125– 127.
Smirnov M, Stepanov V, Korkin A, Electrocapillary phenomena on liquid bismuth in molten alkali bromide mixtures, (Elektrokhimiya) Electrochemistry, 15 (1979) 691–694.
Moelwyn-Hughes E. Physical Chemistry. 2nd ed. Oxford U.K.: Pergamon Press; 1961.
de Gennes PG, Wetting: statics and dynamics, Rev. Mod. Phys. 57 (1985) 827–863. https://doi.org/10.1103/RevModPhys.57.827
Nikolsky BP, Chemist's Handbook. V. 1. GHI: Moscow; 1962. 1072 p.
Moldover MR, Cahn JW, An interface phase transition: complete to partial wetting, Science, 207 (4435) (1980) 1073–1075. https://doi.org/10.1126/science.207.4435.1073
Stepanov V, Dokashenko S, Behavior of the liquid bismuth-molten sodium chloride interface at high anode polarization, Colloid J. 57 (1995) 571–574.
DOI: https://doi.org/10.15826/elmattech.2023.2.020
Copyright (c) 2023 Victor P. Stepanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
