Investigation of Raman spectra and ionic conductivity of composites based on NaClO4 and KClO4 salts obtained by mechanoactivation
Abstract
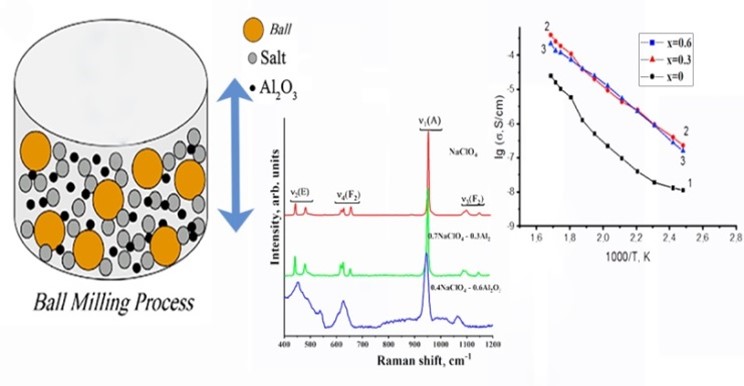
The work is aimed at studying the effect of mechanical activation on the structure and electrical conductivity of the NaClO4 – and KClO4 – basedcomposites. Based on the result of the analysis of the DSC curves of the (1–x) NaClO4 – x Al2O3 and (1–x) KClO4 – x Al2O3 composites measured while heating and cooling the samples, it was established that the enthalpy of phase transitions in them decreased with an increase in the concentration of the nanosized dopant. A complication of all active vibrational contours corresponding to internal vibrations of the molecular anion in the composites with increasing Al2O3 concentration and a shift of the band of the fully symmetric stretching vibration v1 (A) to a low-frequency region was revealed by Raman spectroscopy. Based on electrochemical impedance spectroscopy data, it was determined that for the 0.4NaClO4 – 0.6Al2O3 system subjected to mechanoactivation, the values of specific ionic conductivity increased by two orders of magnitude as compared to that of pure NaClO4, while for the 0.4KClO4 – 0.6Al2O3 system, the values of specific ionic conductivity increased by three orders of magnitude as compared to the initial salt at T = 320 °C.
Keywords
Full Text:
PDFReferences
Liang CC, Conduction Characteristics of the Lithium Iodide‐Aluminum Oxide Solid Electrolytes, J. Electrochem. Soc., 120 (1973) 1289. https://doi.org/10.1149/1.2403248
Ulihin AS, Uvarov NF, Mateyshina YuG, Brezhneva LI, et al., Composite solid electrolytes LiClO4-Al2O3, Solid State Ionics, 177(26–32) (2006) 2787–2790. https://doi.org/10.1016/j.ssi.2006.03.018
Zhang Z, Wang X, Li X, Zhao J, et al., Review on composite solid electrolytes for solid-state lithium-ion batteries, Materials Today Sustainability, 21 (2023) 100316. https://doi.org/10.1016/j.mtsust.2023.100316
Ulihin AS, Uvarov NF, Electrochemical properties of composition solid electrolytes LiClO4-MgO, Russ. J. Electrochem., 45 (2009) 707–710. https://doi.org/10.1134/S1023193509060135
Zou Z, Li Y, Lu Z, Wang D, et al., Mobile Ions in Composite Solids, Chem. Rev., 120(9) (2020) 4169–4221. https://doi.org/10.1021/acs.chemrev.9b00760
Mateyshina Y, Slobodyuk A, Kavun V, Uvarov N, Conductivity and NMR study of composite solid electrolytes CsNO2-A (A = SiO2, Al2O3, MgO), Solid State Ionics, 324 (2018) 196–201. https://doi.org/10.1016/j.ssi.2018.04.026
Uvarov NF, Composite solid electrolytes: recent advances and design strategies, J. Solid State Electrochem., 15(2) (2011) 367–389. https://doi.org/10.1007/s10008-008-0739-4
Xu Z, Zheng L, Chen B, Zhang T, et al., Overview of research on composite electrolytes for solid-state batteries, Energy Storage Science and Technology, 10(6) (2021) 2117–2126. https://doi.org/10.19799/j.cnki.2095-4239.2021.0178
Uvarov NF, Ulihin AS, Mateyshina YuG, Nanocomposite alkali-ion solid electrolytes, Advanced Nanomaterials for Catalysis and Energy, (2022) 393–434. https://doi.org/10.1016/B978-0-12-814807-5.00011-5
Reddy YG, Sekhar MCh, Chary AS, et al., Ion transport studies on Pb(NO3)2:Al2O3 composite solid electrolytes: Effect of dispersoid particle size, IOP Conf. Ser.: Mater. Sci. Eng., 310 (2018) 012160. https://doi.org/10.1088/1757-899X/310/1/012160
Aziam H, Larhrib B, Hakim Ch, Sabi N, et al., Solid-state electrolytes for beyond lithium-ion batteries: A review, Renewable and Sustainable Energy Reviews, 167 (2022) 112694. https://doi.org/10.1016/j.rser.2022.112694
Uvarov NF, Ulihin AS, Slobodyuk AB, Kavun VY, et al., Nanocomposite Solid Electrolytes Based on Lithium Perchlorate, ECS Transactions, 11(31) (2008) 9–17. https://doi.org/10.1149/1.2953501
Leonardi M, Villacampa M, Menéndez JC, Multicomponent mechanochemical synthesis, Chemical Science, 9 (2018) 2042–2064. https://doi.org/10.1039/c7sc05370c
Lee W, Lyon CK, Seo J-H, Lopez-Hallman R, et al., Ceramic–salt composite electrolytes from cold sintering, Advanced Functional Materials, 29(20) (2019) 1807872. https://doi.org/10.1002/adfm.201807872
Gupta SK, Mao Y, A review on molten salt synthesis of metal oxide nanomaterials: Status, opportunity, and challenge, Progress in Materials Science, 117 (2021) 100734. https://doi.org/10.1016/j.pmatsci.2020.100734
Liu X, Fechler N, Antonietti M, Salt melt synthesis of ceramics, semiconductors and carbon nanostructures, Chemical Society Reviews, 42 (2013) 8237–8265. https://doi.org/10.1039/c3cs60159e
Gonzalo-Juan I, Riedel R, Ceramic synthesis from condensed phases, ChemTexts, 2 (2016) 6. https://doi.org/10.1007/s40828-016-0024-6
Ulikhin AS. Transport properties of alkali metal perchlorates and composite solid electrolytes based on them [dissertation]. Novosibirsk (Russia): Russian Academy of Sciences, Siberian Branch, Institute of Solid State Chemistry and Mechanochemistry; 2009. 125 p.
Amirov AM, Suleymanov SI, Gafurov MM, et al., Study of the MNO3–Al2O3 nanocomposites by differential scanning calorimetry, J Therm. Anal. Calorim., 147 (2022) 9283–9290. https://doi.org/10.1007/s10973-022-11256-0
Akhmedov MA, Gafurov MM, Rabadanov KSh, et al., Effect of mechanoactivation on the structure and electrical conductivity in the KNO3-Al2O3 system, Russian Journal of Electrochemistry, 59(8) (2023) 465–473. In press. https://doi.org/10.31857/S0424857023080030
Chen X, Kuroda DG, Ionic conduction mechanism in high concentration lithium ion electrolytes, Chem. Commun., 59 (2023) 1849–1852. https://doi.org/10.1039/D2CC05645C
Ulihin AS, Uvarov NF, Rabadanov KSh, Gafurov MM, et al., Thermal, structural and transport properties of composite solid electrolytes (1-x)(C4H9)4NBF4–xAl2O3, Solid State Ionics, 378 (2022) 115889. https://doi.org/10.1016/j.ssi.2022.115889
Snezhkov VI. Nonlinear inter-ion multiparticle interactions in molten and solid electrolytes [dissertation]. Rostov-on-Don (Russia): North–Caucasian Scientific Center of Higher Education, Rostov-on-Don State Academy of Construction; 1994. 302 p.
Gafurov MM, Rabadanov KSh, High-temperature vibrational spectroscopy of molten electrolytes, Appl. Spectrosc. Rev., 58(7) (2022) 489–508. https://doi.org/10.1080/05704928.2022.2048305
Kalampounias AG, Kirillov SA, Steffen W, Yannopoulos SN, Raman spectra and microscopic dynamics of bulk and confined salol, J. Mol. Struct., 651–653 (2003) 475–483. https://doi.org/10.1016/S0022-2860(03)00128-5
Gafurov MM, Rabadanov KSh, Kubataev ZYu, et al, Vibrational relaxation of perchlorate ion in the (1–x)LiClO4 + xAl2O3 nanocomposites, Bulletin of Dagestan State University. Series 1. Natural Sciences, 34(3) (2019) 102–108. https://doi.org/10.21779/2542-0321-2019-34-3-102-108
Gafurov MM, Rabadanov KS, Ataev MB, Amirov AM, et al., Research of the structure and dynamic interactions of particles in the Li0.42K0.58NO3–R (R = α-Al2O3, γ-Al2O3, SiO2) and (LiNO3–LiClO4) – γ-Al2O3 composites in various temperature conditions and phase states, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 257 (2021) 119765. https://doi.org/10.1016/j.saa.2021.119765
Uvarov N, Ulihin A, Ponomareva V, Kovalenko K, et al., Effect of Pore Filling on Properties of Nanocomposites LiClO4–MIL–101(Cr) with High Ionic Conductivity, Nanomaterials, 12(19) (2022) 3263. https://doi.org/10.3390/nano12193263
Abramczyk H, Paradowska-Moszkowska K, The correlation between the phase transitions and vibrational properties by Raman spectroscopy: Liquid solid β and solid β-solid α acetonitrile transitions, Chem. Phys., 265(2) (2001) 177–191. https://doi.org/10.1016/S0301-0104(01)00271-3
Hosaka T, Kubota K, Kojima H, et al., Highly concentrated electrolyte solutions for 4 V class potassium-ion batteries, Chemical Communications, 54(60) (2018) 8387–8390. https://doi.org/10.1039/c8cc04433c
DOI: https://doi.org/10.15826/elmattech.2024.3.030
Copyright (c) 2024 Zaur Yu. Kubataev, Malik M. Gafurov, Kamil Sh. Rabadanov, Magomed A. Akhmedov, Akhmed M. Amirov

This work is licensed under a Creative Commons Attribution 4.0 International License.

