СОВРЕМЕННЫЕ АНАЛИЗАТОРЫ ДЛЯ ОПРЕДЕЛЕНИЯ АЗОТА МЕТОДОМ КЬЕЛЬДАЛЯ
Аннотация
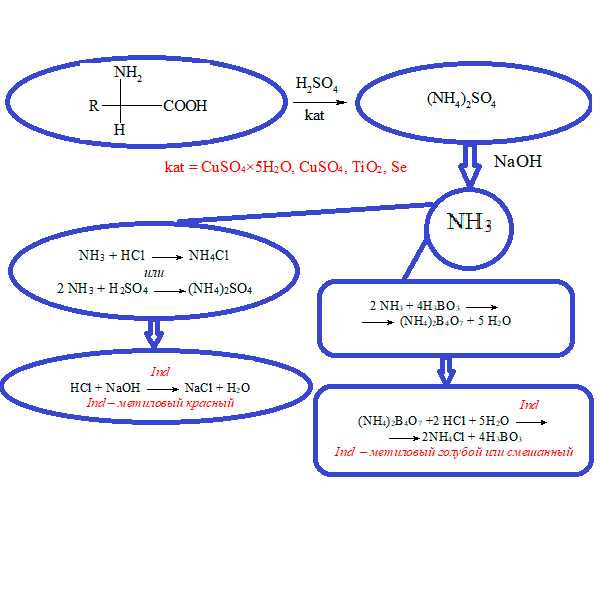
В предлагаемом обзоре рассмотрены основные стадии определения азота методом Кьельдаля: подготовка и кислотная минерализация проб, отгонка и различные варианты (химические и инструментальные) определения аммиака, выделившегося после обработки продуктов минерализации щелочью. Приведены новые технические решения последних лет по совершенствованию и автоматизации отдельных стадий анализа: применение блочных и ИК-дигесторов, существенно повысивших эффективность минерализации образцов и снизивших ее длительность; использование автоматических подъемников минерализованных проб, максимально сокративших работу оператора с тяжелыми пробами и горячими химическими веществами; автосемплеров, обеспечивающих автоматическую передачу минерализованных проб в дистиллятор; автоматизация процессов дистилляции и титрования; использование программного обеспечения для обработки результатов анализа. Систематизированы технические характеристики основных узлов автоматических анализаторов для определения азота методом Кьельдаля – дигесторов, дистилляторов и титраторов, выпускаемых такими известными фирмами, как Buchi (Швейцария), C. Gerhardt, Behr Labor-Technik GmbH и FoodALYT GmbH (Германия), Foss Tecator (Дания, Швеция), VELP Scientifica (Италия), J.P.Selecta (Испания), Hanon Instruments (Китай), ООО ВПК Сибагроприбор и ООО «Вилитек» (Россия). Также приведены данные об основных аналитических характеристиках анализаторов: числе и объеме пробирок (колб), времени минерализации, дистилляции и анализа; массе (объеме) пробы, нижнем пределе обнаружения азота.
Ключевые слова: метод Кьельдаля, кислотная минерализация, отгонка и определение аммиака, автоматический анализатор азота
Полный текст:
PDF (Russian)Литература
REFERENCES
Kjeldahl J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z. Anal. Chem., 1883, vol. 22, pp. 366–382. doi: 10.1007/BF01338151.
Finete V.L.M., Gouvêa M.M., Marques F.F.C., Netto A.D.P. Is it possi-ble to screen for milk or whey protein adulteration with melamine, urea and ammonium sulphate, combining Kjeldahl and classical spectrophotometric methods? Food Chem., 2013, vol. 141, pp. 3649–3655. doi: 10.1016/j.foodchem.2013.06.046.
Schlesier B., Jank H.-W., Schlüter U. Determination of crude protein by Kjeldahl digest and indophenolblue reaction [Rohprotein-Bestimmung durch Kjeldahl-Aufschluß und Indophenoblau-Reaktion]. Die Kulturpflanze, 1984, vol. 32, no. 4, pp. 69–78. doi: 10.1007/BF02002070.
Vinklárková B., Chromý V., Šprongl L., Bittová M., Rikanová M., Ohnútková I., Žaludová L. The Kjeldahl Method as a Primary Reference Pro-cedure for Total Protein in Certified Reference Materials Used in Clinical Chemistry. II. Selection of Direct Kjeldahl Analysis and Its Preliminary Per-formance Parameters. Crit. Rev. Anal. Chem., 2015, vol. 45, pp. 112–118. doi: 10.1080/10408347.2014.892821.
Mason C.J., Coe G., Edwards M., Riby P.G. The use of microwaves in the acceleration of digestion and colour development in the determination of total Kjeldahl nitrogen in soil. Analyst, 1999, vol. 124, no. 11, pp. 1719–1726. doi: 10.1039/a903623g.
Bremner J.M., Tabatabai M.A. Use of an ammonia electrode for deter-mination of ammonium in Kjeldahl analysis of soils. Commun. Soil Sci. Plant Anal., 1972, vol. 3, no. 2, pp. 159–165. doi: 10.1080/00103627209366361.
Sweeney R.A., Gehrke C.W., Rexroad P.R. Fertilizers. Anal. Chem., 1981, vol. 53, pp. 28–38. doi: 10.1021/ac00228a003.
Croll B.T., Tomlinson T., Whitfield C.R.W. Determination of Kjeldahl nitrogen in sewage effluents, trade effluents and sewage sludges using a copper - titanium catalyst. The Analyst, 1985, vol. 110, no. 7, pp. 861–866. doi: 10.1039/AN9851000861.
Sáez-Plaza P., Michałowski T., Navas M.J., Asuero A.G., Wybraniec S. An Overview of the Kjeldahl Method of Nitrogen Determination. Part I. Early History, Chemistry of the Procedure, and Titrimetric Finish. Crit. Rev. Anal. Chem., 2013, vol. 43, pp. 178–223. doi: 10.1080/10408347.2012.751786
Sáez-Plaza P., Navas M.J., Wybraniec S., Michałowski T., Asuero A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sam-ple Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem., 2013, vol. 43, pp. 224–272. doi:10.1080/10408347.2012.751787.
Moore J.C., DeVries J.W., Lipp M., Griffiths J.C., Abernethy D.R. Total protein methods and their potential utility to reduce the risk of food protein adulteration. Compr. Rev. Food Sci. Food Saf., 2010, vol. 9, no. 4, pp. 330–357. doi: 10.1111/j.1541-4337.2010.00114.x
Möller J. Kjeldahl-still going strong. In Focus, 2009, vol. 33, no. 1, pp.14–16.
Persson J. A., Wennerholm M., O’Halloran S. Handbook for Kjeldahl digestion. A Recent Review of the Classical Method with Improvements De-veloped by Foss. 4th ed.; Foss: Hilleroed, Denmark, 2008, 84 p. https://docslide.net/documents/handbook-for-kjeldahl-digestion-562e65c87fcce.html).
McKenzie H.A. The Kjeldahl determination of nitrogen: Retrospect and prospect. TrAC-Trends Anal. Chem., 1994, vol. 13, no. 4, pp.138–144. doi: 10.1016/0165-9936(94)87028-4
Bradstreet R. B. A Review of the Kjeldahl Determination of Organic Ni-trogen. Chem. Rev., 1940, vol. 27, no. 2, pp. 331–350. doi: 10.1021/cr60087a002.
Bradstreet R. B. The Kjeldahl Method for Organic Nitrogen. Academic Press, New York, 1965. рp. 147–168.
Owusu-Apenten R. K. Kjeldahl Method, Quantitative Aminoacid Analy-sis and Combustion Analysis. In Food Protein Analysis: Quantitative Effects on Processing. New York, Marcel Dekker, 2002; Chap. 1, pp. 1–45.
Amin M., Flowers T.H. Evaluation of Kjeldahl digestion method. Jour-nal of Research (Science), 2004, vol. 15, no. 2, pp. 159–179.
Michalski R., Kurzyca I. Determination of nitrogen species (Nitrate, Nitrite and Ammonia Ions) in environmental samples by ion chromatography. Pol. J. Environ. Stud., 2006, vol. 15, no. 1, pp. 5–18.
Stickstoffbestimmung nach Kjeldahl. Available at: https://www.behr-la-bor.de/shop_hacc/50/dokumente/unternehmen/behr_Labor_Technik_Kjeldahl_D.pdf (Accessed20.11.2018).
Gerhardt. Available at: http://www.gerhardt.de/en/product-lines/steam-distillation/distillation-systems-vapodestr/ (Accessed 20.11.2018).
Gerhardt. Available at: http://www.gerhardt.de/en/product-lines/digestion/kjeldathermr-block-digestion-unit/ (Accessed 20.11.2018).
Buchi. Available at: www.buchi.com/kjeldahl (Accessed 20.11.2018).
Velp. Available at: http://www.velp.com/en/service_support/kjeldahl_nitrogen (Accessed 20.11.2018).
Selecta. Available at: http://www.grupo-selecta.com/pdfs/en/cats/catpdf_en_16.pdf (Accessed 20.11.2018).
Selecta. Available at: http://www.grupo-selecta.com/en/catalogo/productos/121/Kjheldahl%20method%20apparatus (Accessed 20.11.2018).
Vilitek. Available at: http://vilitek.ru/products/analizatory-soderzhaniya-belka-zhirov-kletchatki-vlagi/avtomaticheskiy-apparat-keldalya-vilitek-akv-20/ (Accessed 20.11.2018).
MRC Available at: http://www.obrnutafaza.hr/pdf/mrc/proizvodi/Analyzers.pdf (Accessed 20.11.2018).
MRCLAB Available at: https://www.mrclab.com/productsList.aspx?PcatID=1212 (Accessed 20.11.2018).
Hanon Available at: http://www.vicomp.ru/index.php/catalog/kjeldahl/hanon-k1100f (Accessed 20.11.2018).
Hanon Available at: http://www.hanon-rus.ru/index.php/equipment/digestors/hanon-sh220f (Accessed 20.11.2018).
Sibagropribor. Available at: http://sibagropribor.ru/catalog/metod_keldalya/keltrun/#tab1 (Accessed 20.11.2018).
Optimum-lab. Available at: http://www.optimum-lab.ru/product/kompleks-po-opredeleniju-belkaazota-metodom-keldalja/ (Ac-cessed 20.11.2018).
FOSS. Available at: http://foss.su/d/899525/d/laboratornoye_oborudovaniye_foss.pdf (Accessed 20.11.2018).
FOSS. Available at: https://www.fossanalytics.com/ru-ru/products/digestor_2508_2520#Technicalspecificationspot (Accessed 20.11.2018).
Himicheskie analizatory FOSS dlja laboratorij. Available at: http://www.dairynews.ru/upload/iblock/e15/Laboratories%20Foss.pdf (Accessed 20.11.2018).
FoodALYT. Available at: http://foodalyt.de/en/ (Accessed 20.11.2018).
FOSS. Available at: http://foss.su/d/899525/d/laboratornyye_melnitsy_i_gomogenizatory.pdf (Accessed 20.11.2018).
Vilitek. Available at: http://vilitek.ru/products/laboratornye-melnitsy-i-drobilki/) (Accessed 20.11.2018).
Vilitek. Available at: http://vilitek.ru/products/nozhevye-melnitsy-periodicheskogo-deystviya-ekspertnogo-urovnya/laboratornye-melnitsy-dlya-tverdykh-produktov-serii-d/ (Accessed 20.11.2018).
LabFriend. Available at: http://www.labfriend.com.au/kjeldahl-weighing-boat-609-parchment-paper-nitrogen-free; ALBET. Available at: http://www.wiegand.ru/old/catalogs/hahnemuhle_albet_catalog_2010.pdf (Ac-cessed 20.11.2018).
BDL.cz. Available at: http://www.bdl.cz/vyhledavani?q=Kjeldah (Ac-cessed 12.04.2019).
Burns D.T., Stephen W.I. Kjeldahl centenary meeting //Anal. Proc. 1984. vol. 21, no. 6. pp. 210–220. doi: 10.1039/AP9842100210.
Kreusler U., Kissling B., Bamsay W., Loges G., Arnold C., Dafert F.W., Wilfarth H. Über die Bestimmung des Stickstoffs. Z. Anal. Chem., 1885, vol. 24, no. 1, pp. 438–459. doi: 10.1007/BF01366755.
Ashton F.L. Selenium as a catalyst in the Kjeldahl method as applied to soil and grass analysis. The Journal of Agricultural Science, 1936, vol. 26, no. 2, pp. 239–248. https://doi.org/10.1017/S0021859600021924.
Kane P.F. Comparison of HgO and CuSO4 as digestion catalysts in manual Kjeldahl determination of crude protein in animal feeds: collaborative study. J. Assoc. Off. Anal. Chem., 1984. vol. 67, no. 5, pp. 869–877. PubMed: 3680131.
Phelps I.K., Daudt H.W. Investigations of the for Determining Nitrogen Kjeldahle method. J. Assoc. Offic. Agric Chemists, 1920, no. 3, pp. 306–315.
Lauro, M. F. Use of selenium as catalyst in determination of nitrogen by kjeldahl method. Industrial and Engineering Chemistry - Analytical Edition, 1931, vol. 3, no. 4, pp. 401–402. doi: 10.1021/ac50076a033.
Osborne R.A., Wilkie J.B. A Study of the kjeldahle method IV. Metallic catalysts and metallic interferences. J. Assoc. Off. Anal. Chem., 1935, vol 18, pp. 604–609.
Baker P. R. W. The micro-kjeldahl determination of nitrogen. An investi-gation of the effects of added salt and catalysts. Talanta, 1961, vol. 8, no. 2-3, pp. 57–71. doi: 10.1016/0039-9140(61)80040-4.
Middleton G., Stuckey R.E. The Standardjsation of the Digestion Pro-cess in the Kjeldaeil Determination of Nitrogen. J. Pharm. Pharmacol., 1951, vol. 3, no. 1, pp. 829–842. doi: 10.1111/j.2042-7158.1951.tb13127.x.
Rich C.E. The relation of germ content to flour maturation. Cereal Chem. 1934, vol. 11, p. 220
Osborn R.A., Krasnitz A. A study of the Kjeldahl method. J. Assoc. Of-fic. Agr. Chem., 1934, vol. 17, pp. 339–342. https://archive.org/details/in.ernet.dli.2015.26508/page/n347
Kane P.F. CuSO4-TiO2 as Kjeldahl digestion catalyst in manual determi-nation of crude protein in animal feeds. J. Assoc. Off. Anal. Chem., 1986, vol. 69, no. 4, pp. 664–666.
Kane P.F. Comparison of HgO and CuSO4/TiO2 as catalysts in manual Kjeldahl digestion for determination of crude protein in animal feed: collabora-tive study. J. Assoc. Off. Anal. Chem., 1987, vol. 70, no. 5, pp. 907–911.
Jones M.N., Bradshaw H.D. Copper: An alternative to mercury; more effective than zirconium in Kjeldahl digestion of ecological materials. Commu-nications in Soil Science and Plant Analysis, 1989, vol. 20, no. 15–16, pp. 1513-1524. doi: 10.1080/00103628909368165.
Möller J. Protein analysis revisited. In Focus, 2010, vol. 34, no. 2, pp.:22–23.
Gunning J.W. Über eine Modification der Kjeldahl-Methode. Z. analyt. Chem., 1889, vol. 28, no. 1, pp. 188–191. doi: 10.1007/BF01375926.
Chromý V., Vinklárková B., Šprongl L., Bittová M. The Kjeldahl Meth-od as a Primary Reference Procedure for Total Protein in Certified Reference Materials Used in Clinical Chemistry. I. A Review of Kjeldahl Methods Adopt-ed by Laboratory Medicine. Crit. Rev. Anal. Chem., 2015, vol. 45, pp. 106–111. doi: 10.1080/10408347.2014.892820.
Christensen L.M, Fulmer E.I. A Modified Kjeldahl method for the deter-mination of the nitrogen content of yeast. Plant Physiol., 1927, vol. 2, no. 4, pp. 455–460.
Cai J. B., Wang S.-F. Simultaneous Determination of Total Nitrogen and Metal Elements in Tobaccos by High Performance Ion Chromatography. J. Chin. Chem. Soc., 2009, vol. 56, pp. 671–675. doi: 10.1002/jccs.200900100.
Jacobs S., Butterworth D. The Determination of Nitrogen in Biological Materials. C R C Crit. Rev. Anal. Chem., 1978, vol. 7, no. 4, pp. 297–322. doi: 10.1080/10408347808542703.
Hach C.C., Brayton S.V., Kopelove A.B. A Powerful Kjeldahl Nitrogen Method Using Peroxymonosulfuric Acid. J. Agric. Food Chem., 1985, vol. 33, pp. 1117–1123. doi: 10.1021/jf00066a025.
Norton G.A., Adams N.S., Markuszewski R., Braytont S.V. Rapid dis-solution technique for calorimetric determination of nitrogen in coals // Fuel, 1987, vol 66, pp. 996–1001. doi: 10.1016/0016-2361(87)90342-5.
Hilz H., Eilers A., Markle G., Beninga C., Rieger E.M. Kjeldahl Diges-tion: Infrared or Conventional Heating. Nachrichten aus der Chemie, 2012, vol. 60, no. 9, pp. 901–902. doi: 10.1002/nadc.201290317.
Abu-Sarra A., Morris J. S., Koirtyohann S. R. Wet Ashing of Some Bio-logical Samples in a Microwave Oven. Anal. Chem., 1975, vol. 47, no. 8, pp. 1475–1477.
Kubrakova I.V. Microwave radiation in analytical chemistry: The scope and prospects for application. Russian Chemical Reviews, 2002, vol. 71. no. 4. pp. 283–294. doi: 10.1070/RC2002v071n04ABEH000699. https://iopscience.iop.org/article/10.1070/RC2002v071n04ABEH000699/pdf.
Domini C., Vidal L., Cravotto G., Canals A. A simultaneous, direct mi-crowave/ultrasound-assisted digestion procedure for the determination of total Kjeldahl nitrogen. Ultrason. Sonochem., 2009, vol. 16, no. 4, pp. 564–569. doi:10.1016/j.ultsonch.2008.12.006.
Chemat S., Lagha A, Ait Amar H., Chemat F. Ultrasound assisted mi-crowave digestion. Ultrason Sonochem., 2004, vol. 11, no. 1. pp. 5–8. doi: 10.1016/S1350-4177(03)00128-7.
Korn Md., Dos Santos W.P., Korn M., Ferreira S.L. Optimisation of fo-cused-microwave assisted digestion procedure for Kjeldahl nitrogen determina-tion in bean samples by factorial design and Doehlert design. Talanta, 2005, vol. 65, no. 3, pp. 710–715. doi: 10.1016/j.talanta.2004.07.047.
Beljkaš B., Matić J., Milovanović I., Jovanov P., Mišan A., Šarić L. Rap-id method for determination of protein content in cereals and oilseeds: Valida-tion, measurement uncertainty and comparison with the Kjeldahl method. Ac-cred. Qual. Assur., 2010, vol. 15, no. 10, pp. 555–561. doi: 10.1007/s00769-010-0677-6.
Winkler J.W. Beitrag zur titrimetrischen bestimmung des ammoniaks. Angew. Chem. Int. Ed., 1913, vol. 26, no. 31, pp. 231–232.
Martín J., Fernández Sarria L, Asuero A.G. The Kjeldahl Titrimetric Fin-ish: On the Ammonia Titration Trapping in Boric Acid. In book: Advances in Titration Techniques, 2017. pp. 23-58. doi: 10.5772/intechopen.68826.
Ma T. S., Zuazaga G. Determination of Nitrogen by Micro-Kjeldahl Pro-cedure. Ind. Eng. Chem. Anal., Ed. 1942, vol. 14, pp. 280–282.
Marczenko Z., Balcerzak M. Chapter 34 - Nitrogen // Analytical Spec-troscopy Library. 2000. pp. 304–314. doi: 10.1016/S0926-4345(00)80098-X.
Bolleter W.T., Bushman C.J., Tidwell P.W. Spectrophotometric Deter-mination of Ammonia as Indophenol. Anal. Chem., 1961, vol. 33, no. 4, pp. 592–594.
Šraj L.O., Almeida M.I.G.S., Swearer S.E., Kolev S.D., McKelvie I.D. Analytical challenges and advantages of using flow-based methodologies for ammonia determination in estuarine and marine waters (Review). TrAC. Trends Anal. Chem., 2014, vol. 59, pp. 83–92. doi: 10.1016/j.trac.2014.03.012.
Devani M.B., Shishoo C.J., Shah S.A., Suhagia B.N. Spectrophotomet-ric Method for Microdetermination of Nitrogen in Kjeldahl Digest. J. Assoc. Off. Anal. Chem., 1989, vol. 72, pp. 953–956.
Friedman M. Applications of the Ninhydrin Reaction for Analysis of Amino Acids, Peptides, and Proteins to Agricultural and Biomedical Sciences. J. Agric. Food Chem., 2004, vol. 52, no. 3, pp. 385–406. doi: 10.1021/jf030490p.
Quinn J.R., Boisvert J.G., Wood I. Semi-automated ninhydrin assay of Kjeldahl nitrogen. Anal. Biochem., 1974, vol. 58, pp. 609–614. doi: 10.1016/0003-2697(74)90230-9
Danielson N.D., Conroy C. M. Fluorometric determination of hydrazine and ammonia separately or in mixtures. Talanta, 1982, vol. 29, no. 5, pp. 401–404. doi: 10.1016/0039-9140(82)80176-8.
Kitamaki Y., Jin J.-Y., Takeuchi T. Simultaneous determination of inor-ganic nitrogen species by microcolumn ion chromatography. J. Chromatogr. A, 2003, vol. 1003, no. 1-2, pp. 197–202. doi: 10.1016 /S0021-9673(03)00836-7.
Taylor S., Ninjoor V., Dowd D.M., Tappel A.L. Cathepsin B2 measure-ment by sensitive fluorometric ammonia analysis. Anal. Biochem, 1974, vol. 60, no. 1, pp.153–162. doi: 10.1016/0003-2697(74)90140-7.
Goyal S.S., Rains D.W., Huffaker R.C. Determination of Ammonium Ion by Fluorometry or Spectrophotometry after On-Line Derivatization with o-Phthalaldehyde. Anal. Chem., 1988, vol. 60, pp. 175–179. doi: 10.1021/ac00153a016.
Ross J.W., Riseman J.H., Krueger J. A. Potentiometric gas sensing electrodes. Pure Appl. Chem., 1973, vol. 36, no. 4, pp. 473–487. doi: 10.1351/pac197336040473.
Meyerhoff M.E., Fraticelli Y.M. Ion-Selective Electrodes. Anal. Chem., 1982, vol. 54, no. 5, pp. 27–44. doi: 10.1021/ac00242a004.
Verma P., RastogiR.K., Ramakumar K.L. Determination of trace amounts of nitrogen in uranium based samples by ion chromatography (IC) without Kjeldahl distillation. Anal. Chim. Acta, 2007, vol. 596 pp. 281–284. doi:10.1016/j.aca.2007.06.019.
Schwarz J., Kaden H., Pausch G. Development of miniaturized poten-tiometric nitrate- and ammonium selective electrodes for applications in water monitoring. Fresenius' J. Anal. Chem., 2000, vol. 367, no. 4, pp. 396–398. doi: 10.1007/s002160000367.
Jackson D.T., Nelson P.N. Preparation and properties of some ionse-lectivemembranes: A review (Review). J. Mol. Struct., 2019, vol. 1182, no.4, pp. 241–259. doi: 10.1016/j.molstruc.2019.01.050.
Suzuki K., Siswanta D., Otsuka T., Amno T., Ikeda T., Hisamono H., Yashihara R., Ohba S. Design and Synthesis of a More Highly Selective Am-monium Ionophore than Nonactin and its Application as an Ion-Sensing Com-ponent for an Ion-Selective Electrode. Anal. Chem., 2000, vol. 72, pp. 2200-2205.
Chin J., Oh J., Jon S.Y., Park S.H., Walsdorff C., Stranix B., Ghous-soub Lee S. J., Chung H. J., Park S.-M., Kim K. Tuning and dissecting elec-tronic and steric effects in ammonium receptors: Nonactin vs artificial recep-tors. J. Am. Chem. Soc., 2002, vol. 124, no. 19, pp. 5374–5379. doi: 10.1021/ja0174175.
Jonah T.M., Mathivathanan L., Morozov A.N., Mebel A.M., Raptis R.G., Kavallieratos K. Remarkably selective NH4+ binding and fluorescence sensing by tripodal tris(pyrazolyl) receptors derived from 1,3,5-triethylbenzene: Structural and theoretical insights on the role of ion pairing. New J. Chem., 2017, vol. 41, no. 24, pp. 14835–14838. doi: 10.1039/c7nj03213g.
Chin J., Walsdorff C., Stranix B., Oh J., Chung H. J., Park S.-M., Kim K., A Rational Approach to Selective Recognition of NH4+ over K+. Angew. Chem. Int. Ed. 1999, vol. 38, no. 18, pp. 2756–2759. doi: 10.1002/(SICI)1521-3773(19990917)38:18<2756::AID-ANIE2756>3.0.CO;2-6.
Reusch C.F., Cussler E.L. Selective membrane transport. AIChE J., 1973, vol. 19, no. 4, pp. 736–741. doi: 10.1002/aic.690190409.
Kim H.-S., Park H.J., Oh H.J., Koh Y.K., Choi J.-H., Lee D.-H., Ch G.S., Nam H. Thiazole-containing benzo-crown ethers: A new class of ammo-nium-selective ionophores. Anal. Chem., 2000, vol. 72, no. 19, pp. 4683–4688. doi: 10.1021/ac000177b
Späth A., Knig B. Molecular recognition of organic ammonium ions in solution using synthetic receptors (Review). Beilstein J. Org. Chem., 2010, vol. 6, no. 32, pp. 3–111. doi:10.3762/bjoc.6.32.
Graf E., Kintzinger J.P., Lehn J.M., LeMoigne J. Molecular Recogni-tion. Selective Ammonium Cryptates of Synthetic Receptor Molecules Pos-sessing a Tetrahedral Recognition Site. J. Am. Chem. Soc. 1982, vol. 104, pp. 1672–1678. doi: 10.1021/ja00370a037.
Chin J., Oh, J., Jon S.Y., Park S.H., Walsdorff C., Stranix B., Ghous-soub A., Lee S.J., Chung H.J., Park S.-M., Kim K. Tuning and dissecting elec-tronic and steric effects in ammonium receptors: Nonactin vs artificial recep-tors. J. Am. Chem. Soc., 2002, vol. 124, no. 19, pp. 5374–5379. doi: 10.1021/ja0174175.
McGimpsey W.G., Soto E., Driscoll P.F., Nowak C., Benco J.S., Cooper C.G.F., Lambert C.R. 13C NMR study of the ion-binding selectivity of a new ammonium ionophore. Magn. Reson. Chem. 2008, vol. 46, pp. 955–961. doi: 10.1002/mrc.2287.
Benco J.S., Nienaber H.A., McGimpsey W.G. Synthesis of an ammoni-um ionophore and its application in a planar ion-selective electrode. Anal. Chem., 2003, vol. 75, no. 1, pp. 152-156. doi: 10.1021/ac0257851.
Kuswandi B., Nuriman, Verboom W., Reinhoudt D.N. Tripodal recep-tors for cation and anion sensors, Sensors, 2006, vol. 6, no. 8, pp. 978–1017. doi: 10.3390/s6080978.
Schulze M.M., Koch N., Seichter W., Mazik M. Crystalline Ammonium Complexes of Trimethyl- and Triethylbenzene-Based Tripodal Compounds Bearing Pyrazole and Indazole Groups. Eur. J. Org. Chem. 2018, Vol. 2018, no. 31, pp. 4317-4330. doi: 10.1002/ejoc.201800480.
Casadellà A., Schaetzle O., Loos K. Ammonium a cross a selective polymer inclusion membrane: characterization, transport, and selectivity. Mac-romol. Rapid Commun., 2016, vol. 37, pp. 858–864. doi: 10.1002/marc.201600032.
Campins-Falco P., Meseguer-Lloret S., Climent-Santamaria T., Molins-Legua C. A microscale Kjeldahl nitrogen determination for environmental wa-ters. Talanta, 2008, vol. 75, no. 4, pp. 1123–1126. doi:10.1016/j.talanta.2008.01.037.
Růžička J., Hansen E. H. Flow Injection Analyses Part I. A new Concept of Fast Continuous Flow Analysis. Anal. Chim. Acta, 1975, vol. 78, pp. 145–157. doi: 10.1016/S0003-2670(01)84761-9.
Stewart J.W.B., Růžička J., Bergamin F.H., Zagatto E.A. Flow injection analysis. Part III. Comparison of continuous flow spectrophotometry and po-tentiometry for the rapid determination of the total nitrogen content in plant digests. Anal. Chim. Acta, 1976, vol. 81, no. 2, pp. 371–386. doi: 10.1016/S0003-2670(01)82035-3.
Cerdà A., Oms M.T., Forteza R., Cerdà V. Evaluation of flow injection methods for ammonium determination in wastewater samples. Anal. Chim. Ac-ta, 1995, vol. 311, pp. 165–173. doi: 10.1016/0003-2670(95)00182-Y.
Bergamin F. H., Reis B. F., Jacintho A. O., Zagatto E.A.G. Ion exchange in flow injection analysis. Determination of ammonium ions at the m g L21 level in natural waters with pulsed Nessler reagent. Anal. Chim. Acta, 1980, vol. 117, pp. 81–89. doi: 10.1016/0003-2670(80)87007-3.
Wang L., Cardwell T.J., Luque de Castro M.D., Cattrall R.W., Kolev S.D. Determination of ammonia in beers by pervaporation flow injection anal-ysis and spectrophotometric detection. Talanta, 2003, vol. 60, pp. 1269–1275. doi: 10.1016/S0039-9140(03)00235-2.
Wang L., Cardwell T.J., Cattrall R.W., Luque De Castro M.D., Kolev S.D. Pervaporation-flow injection determination of ammonia in the presence of surfactants. Anal. Chim. Acta, 2000, vol. 416, no. 2, pp. 177–184. doi: 10.1016/S0003-2670(00)00901-6.
Kerouel R., Aminot A. Fluorometric determination of ammonia in sea and estuarine waters by direct segmented flow analysis. Mar. Chem., 1997, vol. 57, pp. 267–275. doi: 10.1016/S0304-4203(97)00040-6.
Genfa Z., Dasgupta P.K. Fluorimetric measurement of aqueous ammoni-um ion in a flow injection system. Anal. Chem., 1989, vol. 61, pp. 408–412. doi: 10.1021/ac00180a006.
Liang Y., Yan C., Guo Q., Xu J., Hu H. Spectrophotometric determina-tion of ammonia nitrogen in water by flow injection analysis based on NH3- o-phthalaldehyde -Na2SO3 reaction. Anal. Chem. Res., 2016, vol. 10, pp. 1–8. doi: 10.1016/j.ancr.2016.10.001.
Aminot A., Kerouel R., Birot D. A flow injection-fluorometric method for the determination of ammonium in fresh and saline waters with a view to in situ analyses. Water Res., 2001, vol. 35, no. 7, pp. 1777–1785. doi: 10.1016/S0043-1354(00)00429-2.
Zhu Y., Yuan D., Lin H., Zhou T. Determination of ammonium in sea-water by purge-and-trap and flow injection with fluorescence detection. Anal. Lett., 2016, vol. 49, no. 5, pp. 665–675. doi: 10.1080/00032719.2015.1041027.
Oliveira S.M., Lopes T.I.M.S., T´oth I.V., Rangel A.O.S.S. A multi-commuted flow injection system with a multi-channel propulsion unit placed before detection: Spectrophotometric determination of ammonium, Anal. Chim. Acta, 2007, vol. 600, no. 1-2, рр. 29–34. doi: 10.1016/j.aca.2007.01.019.
Tryzell R., Karlberg B. Calibration methods for determination of ammo-nium and excess acid in Kjeldahl digests by flow injection analysis. Anal. Chimi. Acta, 1997, vol. 343, no. 3, pp. 183–190. doi: 10.1016/S0003-2670(97)00069-X.
Timofeeva I.I., Bulatov A.V., Moskvin A.L., Kolev S.D. A gas-diffusion flow injection method coupled with online solid-liquid extraction for the de-termination of ammonium in solid samples. Talanta, 2015, vol. 142, pp. 140-144. doi: 10.1016/j.talanta.2015.04.051.
Hunter D.A., Uglow R.F. A technique for the measurement of total am-monia in small volumes of seawater and hemolymph. Ophelia, 1993, vol. 37, pp. 31–40. doi: 10.1080/00785326.1993.10430375.
Lima J.F.C.C., Delerue-Matos C., Carmo Vaz M. Flow-injection analysis of Kjeldahl nitrogen in milk and dairy products by potentiometric detection. Anal. Chim. Acta, 1999. vol. 385, no. 1-3, pp. 6–9. doi: 10.1016/S0003-2670(98)00687-4.
Bulatov А.V., Ivasenko P.A., Moskvin A.L., Moskvin L.N. Stepwise in-jection potentiometric determination of ammonium-ions in waters. J. Flow In-jection Anal., 2009, vol. 26, no. 1, pp. 49–52. doi: https://doi.org/10.24688/jfia.26.1_49.
Pasquini C., De Faria L.C. Flow-injection determination of ammonia in kjeldahl digests by gas diffusion and conductometry. Anal. Chim. Acta, 1987, vol. 193, no. 19. pp. 19–27. doi: 10.1016/S0003-2670(00)86134-6.
Junsomboon J., Jakmunee J. Flow injection conductometric system with gas diffusion separation for the determination of Kjeldahl nitrogen in milk and chicken meat. Anal. Chim. Acta, 2008, vol. 627, no. 2, pp. 232–238. doi: 10.1016/j.aca.2008.08.012.
Yanu P., Jakmunee J. Down scaled Kjeldahl digestion and flow injection conductometric system for determination of protein content in some traditional northern Thai foods. Food Chem., 2017, vol. 230, no. 1, pp. 572–577. https://doi.org/10.1016/j.foodchem.2017.02.142.
Watson R.J., Butler E.C.V., Clementson L.A., Berry K.M. Flow-injection analysis with fluorescence detection for the determination of trace levels of ammonium in seawater. Environ. Monit., 2005, vol. 7, pp. 37–42. doi: 10.1039/b405924g.
Amornthammarong N., Zhang J.-Z., Ortner P.B. An autonomous batch analyzer for the determination of trace ammonium in natural waters using fluo-rometric detection. Analytical Methods, 2011, vol. 3, no. 7, pp. 1501-1506. doi: 10.1039/c1ay05095h.
Small H. Ion Chromatography. New York, Plenum Publishing, 1989, 276 p. doi: 10.1007/978-1-4899-2542-8.
Michalski R. Ion Chromatography Applications in Waste water Analysis // Separations, 2018, vol. 5, no. 16. doi: 10.3390/separations5010016.
Michalski R. Applications of Ion Chromatography for the Determination of Inorganic Cations. Crit. Rev. Anal. Chem., 2009, vol. 39, no. 4, pp. 230–250. https://doi.org/10.1080/10408340903032453.
Small H., Stevens T.S., Bauman W.C. Novel Ion Exchange Chromato-graphic Method Using Conductometric Detection. Anal. Chem., 1975, vol. 47, pp. 1801–1806. doi: 10.1021/ac60361a017.
Fritz J.S., Gjerde D.T., Pohlandt C. Ion Chromatography, Dr. Alfred Huthig Verlag, Heidelberg-Basel-New York. 1982.
Salt Converter-Cation Electrolytically Regenerated Suppressor 500 (Di-onex SC-CERS 500) 031849 Revision 04 • December 2016 Thermo Fisher Scientific Inc. https://assets.thermofisher.com/TFS-Assets/CMD/manuals/Man-031849-IC-SC-CERS-500-Man031849-EN.pdf.
Srinivasan K., Bhardwaj S., Lin R., Pohl C. Suppressor design and de-tection for ion chromatography. In book: Applications of Ion Chromatography for Pharmaceutical and Biological Products. New York, NY: Wiley, 2012. pp. 91-105. doi: 10.1002/9781118147009.ch4.
Trevani L.N., Roberts J.C., Tremaine P.R. Copper (II)-ammonia com-plexation equilibria in aqueous solutions at temperatures from 30 to 250°C by visible spectroscopy. J. Solution Chem., 2001, vol. 30, no. 7, pp. 585-622. doi: 10.1023/A:1010453412802.
Jackson P.E, Krol J., Heckenberg A.L., Mientjes M., Staal W. Determina-tion of total nitrogen in food, environmental and other samples by ion chroma-tography after Kjeldahl digestion. J. Chromatogr., 1991, vol. 546, pp. 405-410. doi: 10.1016/S0021-9673(01)93039-0.
Pontes F.V.M., Carneiro M.C., Vaitsman D.S., da Rocha G.P., da Silva L.I.D., Neto A.A., Monteiro M.I.C. A simplified version of the total Kjeldahl nitrogen method using an ammonia extraction ultrasound-assisted purge-and-trap system and ion chromatography for analyses of geological samples. Anal Chim Acta. 2009; vol. 632,:pp. 284-288. doi: 10.1016/j.aca.2008.11.011.
Wang H., Pampati N., McCormick W.M., Bhattacharyya L. Protein Ni-trogen Determination by Kjeldahl Digestion and Ion Chromatography. J. Pharm. Sci., 2016, vol. 105, no. 6, pp. 1851–1857. https://doi.org/10.1016/j.xphs.2016.03.039.
Moldoveanu S. Chromatographie détermination of total nitrogen follow-ing the kjeldahl oxidation. J. Chromatogr. Sci., 1988, vol. 26, no. 1, pp. 12–14. doi: 10.1093/chromsci/26.1.12.
Krotz L., Cicerci E., Giazzi G. Protein Determination in Cereals and Seeds. Food Quality 2008, 15 (4), pp. 37–39.
Rowland S.J. The protein distribution in normal and abnormal milk. J. Dairy Res., 1938, vol. 357, no. 9, pp. 47–57. doi: 10.1017/S0022029900002302.
Gao P., Li Z., Zan L., Yue T., Shi B. A non-protein nitrogen index for discriminating raw milk protein adulteration via the Kjeldahl method. Analyti-cal Methods, 2015, vol. 7, pp. 9166–9170. doi: 10.1039/c5ay01422k.
DeVries J.W., Greene G.W., Payne A., Zbylut S., Scholl P.F., Wehling P., Evers J.M., Moore J.C. Non-protein nitrogen determination: A screening tool for nitrogenous compound adulteration of milk powder. International Dairy Journal, 2017, vol. 68, pp. 46–51. https://doi.org/10.1016/j.idairyj.2016.12.003.
Sinaga S.M., Lubis I.Y., Silalahi J. Analysis of Total Protein and Non Pro-teinnitrogen in Pakkat (Calamus caesius Blume.) as a Traditional Food of Mandailing Natal by using Kjeldahl Method. Int. J. Pharm. Tech Res. 2016, vol. 9, no. 12, pp. 543–549.
Handbook of Pharmaceutical Excipient / Edited by Rowe R.C., Sheskey P.J., Owen S.C. London: APhA/Pharmaceutical Press, 2009. 888 p. Available at: https://trove.nla.gov.au/version/208133392 (Accessed 20.11.2018).
Scott L.D. Rapid methods for the quantitative determination of total pro-tein and non-protein nitrogen in human and cow's milk. Biochem. J., 1934, vol. 28, pp. 1193–1197.
Courtney A.M., Brown A. The protein and non-protein fractions of some samples of woman's milk. Archives of Disease in Childhood, 1930, vol. 5, pp. 36–41.
Silanikove N., Perevolotsky A., Provenza F.D. Use of tannin-binding chemicals to assay for tannins and their negative postingestive effects in rumi-nants. Animal Feed Science and Technology, 2001, vol. 91, pp. 69–81. doi: 10.1016/S0377-8401(01)00234-6.
Turley D.J., Kelly M.T., Smyth M.R. High-performance liquid chroma-tographic method for the comparison of tanning capacity of tannic acid batch-es used in the manufacture of pregnancy testing kits. J. Chromatogr. A, 1990, vol. 513, pp. 263–269. doi: 10.1016/S0021-9673(01)89443-7.
Chang S.K.C. Protein Analysis. In: Food Analysis, 2010, pp. 133–146. doi: 10.1007/978-1-4419-1478-1_9.
Krul E.S. Calculation of Nitrogen-to-Protein Conversion Factors: A Re-view with a Focus on Soy Protein JAOCS. J. Am. Oil Chem. Soc., 2019, doi: 10.1002/aocs.12196. (Article in press).
Ссылки
- На текущий момент ссылки отсутствуют.
