Morphological and structural features of the CdxPb1−xS films obtained by CBD from ethylenediamine-citrate bath
Abstract
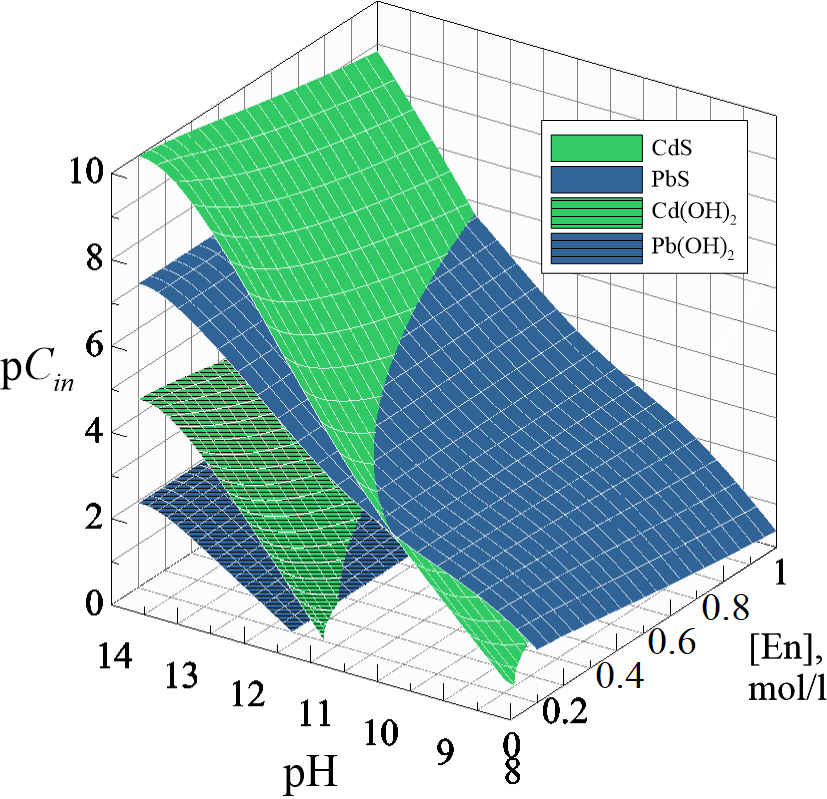
The calculating of ionic equilibria in the system «Pb(CH3COO)2 - CdCl2 - Na3C6H5O7 - (NH3)2(CH2)2 - N2H4CS» allowed us to find conditions and concentration regions of PbS and CdS co-deposition. The determined conditions provided the CBD obtaining of CdxPb1−xS (0 ≤ x ≤ 0.033) substitutional solid solutions films with a cubic structure B1 (space group Fm ) with the grains preferred orientation (200). We established the evolution of the surface morphology of the synthesized films from cubic crystallites to hierarchical structure of globular aggregates by scanning electron microscopy. A quantitative analysis of diffraction patterns showed a decrease of microstrains in CdxPb1−xS films by a about factor of 3 with an increase of the cadmium chloride concentration in the reaction mixture from 0.005 to 0.14 mol/l. The excess of the cadmium content, established by EDX analysis, in the studied films as compared to its content in the solid solution is associated with the additional formation of the amorphous CdS phase up to 72 mol %.
A Corrigendum is available for this article at https://doi.org/10.15826/chimtech.2021.8.2.12.
Keywords
Full Text:
PDFReferences
Suryavanshi KE, Dhake RB, Patil AM, Sonawane MR. Growth mechanism and transport properties of chemically deposited Pb Cd S thin film’s photoelectrochemical (PEC) solar cell. Optik. 2020;165008. doi:10.1016/j.ijleo.2020.165008
Ounissi A, Ouddai N, Achour S. Optical characterisation of chemically deposited Pb(1−x)CdxS films and a Pb1−xCdxS(n)/Si(p) heterojunction. Eur Phys J Appl Phys. 2007;37(3):241−5. doi:10.1051/epjap:2007034
Maskaeva LN, Markov VF, Porkhachev MYu, Mokrousova АО. Thermal and radiation stability IR-detectors based on films of solid solutions CdxPb1−xS. Pozharovzryvobezopasnost [Fire and Explosion Safety]. 2015;24(9):67-73. Russian. doi:10.18322/PVB.2015.24.09.67-73
Pentia E, Draghici V, Sarau G, et al. Structural, electrical, and photoelectrical properties of CdxPb1−xS thin films prepared by chemical bath deposition. J Electrochem Soc. 2004;151(11):G729−33. doi:10.1149/1.1800673
Bezdetnova AE, Markov VF, Maskaeva LN, et al. Determination of nitrogen dioxide by thin-film chemical sensors based on CdxPb1–xS. J Anal Chem. 2019;74(12):1256−62. doi:10.1134/S1061934819120025
Au GHT, Shih WY, Tseng SJ, Shih WH. Aqueous CdPbS quantum dots for near-infrared imaging. Nanotechnology. 2012;23(27):275601(1-9). doi:10.1088/0957-4484/23/27/275601
Tan GL, Liu L, Wu W. Mid-IR band gap engineering of CdxPb1−xS nanocrystals by mechanochemical reaction. AIP Advances. 2014;4(6):067107(1-11). doi:10.1063/1.4881878
Nichols PL, Liu Z, Yin L, et al. CdxPb1–xS alloy nanowires and heterostructures with simultaneous emission in mid-infrared and visible wavelengths. Nano Lett. 2015;15(2):909−16. doi:10.1021/nl503640x
Rabinovich E, Wachtel E, Hodes G. Chemical bath deposition of single-phase (Pb,Cd)S solid solutions. Thin Solid Films. 2008;517(2):737−44. doi:10.1016/j.tsf.2008.08.162
Barote M, Yadav A, Masumdar E. Effect of deposition parameters on growth and characterization of chemically deposited Cd1-xPbxS thin films. Chalcogenide Lett. [Internet]. 2021[cited 2021];8(2):129−38. Available from: https://chalcogen.ro/index.php/journals/chalcogenide-letters/11-cl/126-volume-8-number-2-february-2011
Maskaeva, LN, Kutyavina AD, Markov VF, et al. Features of the formation of thin films of supersaturated CdxPb1–xS solid solutions by chemical bath deposition. Russ J Gen Chem. 2018;88(2):295–304. doi:10.1134/S1070363218020172
Maskaeva LN, Pozdin AV, Markov VF, et al. Effect of the substrate nature on the CdPbS film composition and mechanical stresses at the “film–substrate” interface. Semiconductors. 2020;54:1567–76. doi:10.1134/S1063782620120209
Rajathi S, Kirubavathi K, Selvaraju K. Preparation of nanocrystalline Cd-doped PbS thin films and their structural and optical properties. J of Taibah University for Science. 2017;11(6):1296−305. doi:10.1016/j.jtusci.2017.05.001
Suryavanshi KE, Dhake RB, Patil AM, Sonawane MR. Growth mechanism and transport properties of chemically deposited PbCdS thin film’s photoelectrochemical (PEC) solar cell. Optik. 2020;165008. doi:10.1016/j.ijleo.2020.165008
Ahmad SM, Kasim SJ, Latif LA. Effects of thermal annealing on structural and optical properties of nanocrystalline CdxPb1-xS thin films prepared by CBD. Jordan Journal of Physics [Internet]. 2016[cited 2021];9(2):113−22. Available from: http://journals.yu.edu.jo/jjp/JJPIssues/Vol9No2pdf2016/7.pdf
Suryavanshi KE, Dhake RB, Patil AM, et al. Structural properties of PbxCd1−xS thin films prepared by chemical bath deposition technique. Int J Adv Res [Internet]. 2014[cited 2021];2(6):491−3. Available from: http://www.journalijar.com/uploads/926_IJAR-3389.pdf
Thangavel S, Ganesan S, Chandramohan S, et al. Band gap engineering in PbS nanostructured thin films from near-infrared down to visible range by in situ Cd-doping. J Alloys Comp. 2010;495(1):234237. doi:10.1016/j.jallcom.2010.01.135
Deo SR, Singh AK, Deshmukh L, et al. Studies on structural, morphological and optical behavior of chemically deposited Cd0.5Pb0.5S thin films. Optik. 2015;126(20):2311–17. doi:10.1016/j.ijleo.2015.05.130
Lurie YuYu. Spravochnik po analiticheskoy khimii [Analytical chemistry handbook]. Мoscow: Khimiya; 1989. 488 p. Russian.
Maskaeva LN, Voronin BI, Mostovshchikova EV, et al. Chemical bath deposited CdxPb1-xS solid solution films: composition, structure, optical properties. Thin Solid Films. 2021;718(12):138468. doi:10.1016/j.tsf.2020.138468
Nikolsky BP. Spravochnik khimika. Tom 3 [Chemist's handbook. Volume 3]. Leningrad: Khimiya; 1971. 1008 p. Russian.
Rietveld HM. A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr. 1969;2(2):65−71. doi:10.1107/S0021889869006558
Bush DL, Post JE. A survey of using programs for the Rietveld profile refinement. Reviews in mineralogy. 1990;20:369−74. doi:10.1180/claymin.1990.025.4.12
Rodriges-Carvajal J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B. 1993;192:55−69. doi:10.1016/0921-4526(93)90108-I
Williamson GK, Hall WH. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953;1:22−31. doi:10.1016/0001-6160(53)90006-6
Maskaeva LN, Markov VF, Vaganova IV, et al. Films of supersaturated CdxPb1−xS solid solutions: composition prognostication, chemical synthesis, microstructure. Russ J Appl Chem. 2017;90(5):691–700. doi:10.1134/S1070427217050044
Vinogradova TV, Markov VF, Maskaeva LN. Temperature dependence of constants of thiourea hydrolytic decomposition and cyanamide. Stepwise ionization. Russ J Gen Chem. 2010;80:2341–6. doi:10.1134/S1070363210110198
Maskaeva LN, Markov VF, Forostyanaya NA, et al. Kinetic aspects of hydrochemical deposition of cadmium sulfide from solutions with diverse ligand backgrounds. Russ J Gen Chem. 2016;86(10):2273−81. doi:10.1134/S1070363216100054
Lee S-M, Cho S-N, Cheon J. Anisotropic shape control of colloidal inorganic nanocrystals. Adv Mater. 2003;15(5):441−4. doi:10.1002/adma.200390102
Forostyanaya NA, Maskaeva LN, Markov VF. Influence of the ligand nature on the boundary conditions of the formation and the morphology of nanocrystalline cadmium sulfide films. Russ J Gen Chem. 2015;85:2513–19. doi:10.1134/S1070363215110031
Markov VF, Maskaeva LN. Nucleation and mechanism of metal sulfide film growth using deposition by thiocarbamide. Russ Chem Bull. 2014;63(7):1523−32. doi:10.1007/s11172-014-0630-7
Bugaenko LT, Ryabykh SM, Bugaenko AL. A nearly complete system of average crystallographic ionic radii and its use for determining ionization potentials. Moscow Univ Chem Bull. 2008;63:303–17. doi:10.3103/S0027131408060011
Kobayashi T, Susa K, Taniguchi S. Preparation and semiconductive properties of rock salt type solid solution systems, Cd1−xMxS (M = Sr, Ca, Mg, Pb, Sn). J Phys Chem Solids. 1979;40:781−5. doi:10.1016/0022-3697(79)90160-4
Corll JA. Recovery of the high‐pressure phase of cadmium sulfide. J Appl Phys. 1964;35(10):3032–3. doi:10.1063/1.1713151
Rooymans CJM. Structure of the high pressure phase of CdS, CdSe, and InSb. Phys Lett. 1963;4:186−7. doi:10.1016/0031-9163(63)90356-1
Susa K, Kobayashi T, Taniguchi S. High-pressure synthesis of rock-salt type CdS using metal sulfide additives. J Solid State Chem. 1980;33:197−202. doi:10.1016/0022-4596(80)90120-6
Vegard L. Die konstitution der mischkristalle und die raumfüllung der atome. Zeitschrift für Physik. 1921;5:17−26. doi:10.1007/BF01349680
Denton AR, Ashcroft NW. Vegard’s law. Phys Rev A. 1991;43:3161−4. doi:10.1103/PhysRevA.43.3161
Shelimova LE, Tomashik VN, Gritsyv VI. Diagrammy sostoyaniya v poluprovodnikovom materialakh (sistemy na osnove khal'kogenidov Si, Ge, Sn, Pb) [State diagrams in semiconductor materials science (systems based on chalcogenides Si, Ge, Sn, Pb]. Moscow: Nauka; 1991. 368 p. Russian.
DOI: https://doi.org/10.15826/chimtech.2021.8.2.10
Copyright (c) 2021 A.D. Kutyavina, L.N. Maskaeva, V.I. Voronin, I.А. Anokhina, V.F. Markov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014-2024
ISSN 2411-1414 (Online)
Copyright Notice







