Electrophoretic deposition of coatings and bulk compacts using magnesium-doped aluminum oxide nanopowders
Abstract
Keywords
Full Text:
PDFReferences
He J, Avnir D, Zhang L. Sol-gel derived alumina glass: Mechanistic study of its structural evolution. Acta Mater. 2019;174:418–26. doi:10.1016/j.actamat.2019.05.062
Kelekanjeri VG, Carter WB, Hampikian JM. Deposition of α-alumina via combustion chemical vapor deposition. Thin Solid Films. 2006;515(4):1905–11. doi:10.1016/j.tsf.2006.07.033
Ogita Y, Saito N. Formation of alumina film using alloy catalyzers in catalytic chemical vapor deposition. Thin Solid Films. 2015;575:47–51. doi:10.1016/j.tsf.2014.10.022
Boidin R, Halenkovič T, Nazabal V, Beneš L, Němec P. Pulsed laser deposited alumina thin films. Ceram Int. 2016;42(1):1177–82. doi:10.1016/j.ceramint.2015.09.048
Korhonen H, Syväluoto A, Leskinen JTT, Lappalainen R. Optically transparent and durable Al2O3 coatings for harsh environments by ultra short pulsed laser deposition. Opt Laser Technol. 2018;98:373– 84. doi:10.1016/j.optlastec.2017.07.050
Kishida S, Ju D, He H, Li Y. Coating of γ-Al2O3 on the stainless steel substrate by electrophoretic deposition method. J Environ Sci. 2009;21:S112–5. doi:10.1016/S1001-0742(09)60051-6
Novak S, König K. Fabrication of alumina parts by electrophoretic deposition from ethanol and aqueous suspensions. Ceram Int. 2009;35(7):2823–9. doi:10.1016/j.ceramint.2009.03.033
Song G, Xu G, Quan Y, Yuan Q, Davies PA. Uniform design for the optimization of Al2O3 nanofilms produced by electrophoretic deposition. Surf Coat Technol. 2016;286:268–78. doi:10.1016/j.surfcoat.2015.12.039
Takao Y, Hotta T, Naito M, Shinohara N, Okumiya M, Uematsu K. Microstructure of alumina compact body made by slip casting. J Eur Ceram Soc. 2002;22(4):397–401. doi:10.1016/S0955-2219(01)00307-7
Ivanov VV, Paranin SN, Khrustov VR. Nanostructured ceramics based on aluminum and zirconium oxides produced using magnetic pulsed pressing. Phys Metals Metallogr. 2002;94:S98–S106.
Kaygorodov A, Rhee C, Kim W, Ivanov V, Paranin S, Spirin A, Khrustov V. Nozzles from Alumina Ceramics with Submicron Structure Fabricated by Radial Pulsed Compaction. Mater Sci Forum. 2007;534-536:1053–6. doi:10.4028/www.scientific.net/MSF.534-536.1053
Promdej C, Pavarajarn V, Wada S, Wasanapiarnpong T, Charinpanitkul T. Effect of hot isostatically pressed sintering on microstructure of translucent alumina compact. Curr Appl Phys. 2009;9(5):960–6. doi:10.1016/j.cap.2008.09.011
Okuma G, Watanabe S, Shinobe K, Nishiyama N, Takeuchi A, Uesugi K, Tanaka S, Wakai F. 3D multiscale-imaging of processing-induced defects formed during sintering of hierarchical powder packings. Sci Rep. 2019;9:11595. doi:10.1038/s41598-019-48127-y
Corni I, Ryan MP, Boccaccini AR. Electrophoretic deposition: From traditional ceramics to nanotechnology. J Eur Ceram Soc. 2008;28(7):1353–67. doi:10.1016/j.jeurceramsoc.2007.12.011
Pikalova E, Kalinina E. Place of electrophoretic deposition among thin-film methods adapted to the solid oxide fuel cell technology: A short review. J of Energy Prod and Mgm. 2019;4(1):1–27. doi:10.2495/EQ-V4-N1-1-27
Pikalova EYu, Kalinina EG. Electrophoretic deposition in the solid oxide fuel cell technology: Fundamentals and recent advances. Renew Sust Energ Rev. 2019;116:109440. doi:10.1016/j.rser.2019.109440
Kalinina EG, Pikalova EYu. New trends in the development of electrophoretic deposition method in the solid oxide fuel cell technology: theoretical approaches, experimental solutions and development prospects. Russ Chem Rev. 2019;88(12):1179–219. doi:10.1070/RCR4889
König K, Novak S, Boccaccini AR, Kobe S. The effect of the particle size and the morphology of alumina powders on the processing of green bodies by electrophoretic deposition. J Mater Process Technol. 2010;210(1):96–103. doi:10.1016/j.jmatprotec.2009.08.007
Xu P, Mujumdar AS, Yu B. Drying-Induced Cracks in Thin Film Fabricated from Colloidal Dispersions. Drying Technology. 2009;27(5):636–52. doi:10.1080/07373930902820804
Santanach Carreras E, Chabert F, Dunstan DE, Franks GV. Avoiding “mud” cracks during drying of thin films from aqueous colloidal suspensions. Journal of Colloid and Interface Science. 2007;313(1):160–8. doi:10.1016/j.jcis.2007.03.076
Koltsov I, Smalc-Koziorowska J, Prześniak-Welenc M, Małysa M, Kimmel G, McGlynn J, Ganin A, Stelmakh S. Mechanism of Reduced Sintering Temperature of Al2O3–ZrO2 Nanocomposites Obtained by Microwave Hydrothermal Synthesis. Materials. 2018;11(5):829. doi:10.3390/ma11050829
Lewis JA. Colloidal Processing of Ceramics. J Am Ceram Soc. 2000;83(10):2341–59. doi:10.1111/j.1151-2916.2000.tb01560.x
Kotov YA. Electric Explosion of Wires as a Method for Preparation of Nanopowders. J Nanopart Res. 2003;5:539–50. doi:10.1023/B:NANO.0000006069.45073.0b
Kotov YA. The electrical explosion of wire: A method for the synthesis of weakly aggregated nanopowders. Nanotechnol Russia. 2009;4:415–24. doi:10.1134/S1995078009070039
Safronov AP, Kalinina EG, Smirnova TA, Leiman DV, Bagazeev AV. Self-stabilization of aqueous suspensions of alumina nanoparticles obtained by electrical explosion. Russ J Phys Chem А. 2010;84:2122–7. doi:10.1134/S0036024410120204.
Harun Z, Ismail NF, Badarulzaman NA. Effect of MgO Additive on Microstructure of Al2O3. Adv Mat Res. 2012;488-489:335–9. doi:10.4028/www.scientific.net/AMR.488-489.335
Pikalova EYu, Kalinina EG. Approaches to improving efficiency of solid oxide fuel cells based on ceramic membranes with mixed conductivity. Russ Chem Rev. 2021;90 In Press. doi:10.1070/RCR4966
Maca K, Hadraba H, Cihlar J. Electrophoretic deposition of alumina and zirconia: I. Single-component systems. Ceram Int. 2004;30(6):843–51. doi:10.1016/j.ceramint.2003.09.021
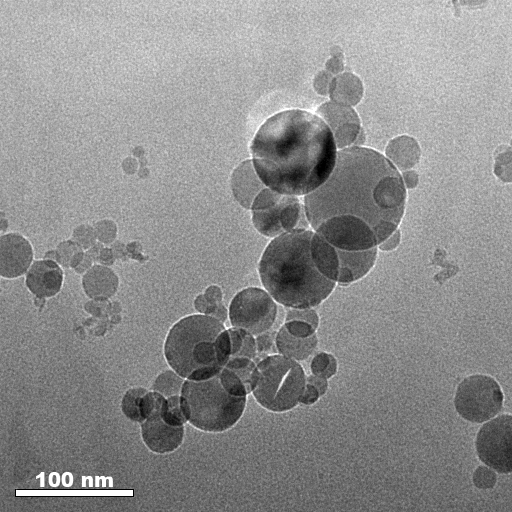
Kotov YuA, Beketov IV, Azarkevich EI, Murzakaev AM. Synthesis of Nanometer-Sized Powders of Alumina Containing Magnesia. In: Proceedings of the Ninth CIMTEC-World Ceramic Congress “Ceramics: Getting into the 2000s”, 1998 Jun 14–19; Florence, Italy. p. 277–84.
Bhattacharjee S. DLS and zeta potential – What they are and what they are not? J Control Release. 2016;235:337–51. doi:10.1016/j.jconrel.2016.06.017
Aznam I, Mah JCW, Muchtar A, Somalu MR, Ghazali MJ. A review of key parameters for effective electrophoretic deposition in the fabrication of solid oxide fuel cells. Univ Sci A. 2018;19:811–23. doi:10.1631/jzus.A1700604
DOI: https://doi.org/10.15826/chimtech.2021.8.2.06
Copyright (c) 2021 Elena G. Kalinina, Darya S. Rusakova, Elena Yu. Pikalova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014-2024
ISSN 2411-1414 (Online)
Copyright Notice







