ВЛИЯНИЕ ПРИРОДЫ МОДИФИКАТОРА НА ЭФФЕКТИВНОСТЬ КОНЦЕНТРИРОВАНИЯ РУТИНА И КВЕРЦЕТИНА НА НАНОЧАСТИЦАХ МАГНЕТИТА
Аннотация
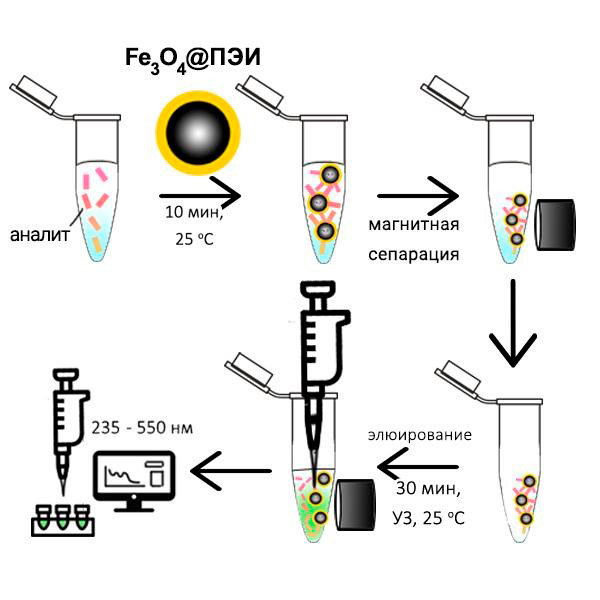
Методом химического соосаждения синтезированы магнитные наночастицы (МНЧ) магнетита, поверхность которых модифицирована диоксидом кремния, диоксидом кремния и полиэтиленимином и только полиэтиленимином. Полученные МНЧ охарактеризованы методами электрофоретического рассеяния света и просвечивающей электронной микроскопии. Показано, что на величину и знак дзета-потенциала МНЧ влияют природа модификатора и рН раствора. Изучено влияние рН, количества сорбента, времени сорбции, способа перемешивания раствора и найдены оптимальные условия сорбции кверцетина и рутина. Установлено, что сорбция указанных флавоноидов количественно происходит на магнетите, модифицированном как SiO2@ПЭИ так и только ПЭИ, протекает за 10 мин, однако степень извлечения выше на МНЧ, модифицированных ПЭИ, которая для кверцетина и рутина составляет 98 % и 86 %, соответственно. Показано, что степень извлечения кверцетина и рутина при десорбции 4 мл 0.1 М NaOH в течение 20 минут составляет 62 и 56 процентов, соответственно.
Ключевые слова: магнетит, наночастицы, магнитная твердофазная экстракция, флавоноиды, кверцетин, рутин, концентрирование
Полный текст:
PDF (Russian)Литература
REFERENCES
Agati G., Azzarello E., Pollastri S., Tattini M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Science, 2012, vol. 196, pp. 67–76. doi: 10.1016/j.plantsci.2012.07.014.
Pietta P.G. Flavonoids as antioxidants. Journal of Natural Products, 2000, vol. 63, no. 7, pp. 1035-1042. doi: 10.1021/np9904509.
Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016, vol. 5, no. 47, pp. 1-15. doi:10.1017/jns.2016.41.
Malešev D., Kunti V. Investigation of metal–flavonoid chelates and the determination of flavonoids via metal– lavonoidcomplexing reactions. Journal of the Serbian Chemical Society, 2007, vol. 72, no. 10, pp. 921–939. doi: 10.2298/JSC0710921M.
Corradini E., Foglia P., Giansanti P., Gubbiotti R., Samperi R. Flavonoids: chemical properties and analytical methodologies of identification and quantitation in foods and plants. Natural Product Research, 2011, vol. 25, no. 5, pp. 469–495. doi: 10.1080/14786419.2010.482054.
Ziyatdinova G.K., Budnikov G.K. Natural phenolic antioxidants in bioanalytical chemistry: state of the problem and development prospects. Russian Chemical Reviews, 2015, vol. 84, no. 2, pp. 194-224. DOI: 10.1070/RCR4436
Zenkevich I. G., Gushchina R. V. Determination of dissociation constants of species oxidizable in aqueous solution by air oxygen on an example of quercetin. Journal of Analytical Chemistry, 2010, vol. 65, no. 4, pp. 371–375. DOI: 10.1134/S1061934810040064
Boots A. W., Haenen G. R. M. M., Bast A. Health effects of quercetin: from antioxidant to nutraceutical. European Journal of Pharmacology, 2008, vol. 585, no. 2-3, pp. 325-337. doi: 10.1016/j.ejphar.2008.03.008.
Yang J., Guo J., Yuan J. In vitro antioxidant properties of rutin. LWT - Food Science and Technology, 2008, vol. 41, no. 6, pp. 1060-1066. doi: 10.1016/j.lwt.2007.06.010.
Kochetova, M. V., Semenistaya, E. N., Larionov, O. G., & Revina, A. A. Determination of biologically active phenols and polyphenols in various objects by chromatography. Russian Chemical Reviews, 2007, vol. 76, no. 1, pp. 79-90. doi: 10.1070/RC2007v076n01ABEH003632
Liu E.H., Qi L.W., Cao J., Li P., Li C.Y., Peng Y.B. Advances of Modern Chromatographic and Electrophoretic Methods in Separation and Analysis of Flavonoids. Molecules, 2008, vol. 13, no. 10, pp. 2521-2544. doi: 10.3390/molecules13102521.
Ignat I., Volf I., Popa V.I. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chemistry, 2011, vol. 126, no. 4, pp. 1821-1835. doi: 10.1016/j.foodchem.2010.12.026.
Shevlyakova O.A., Ihalajnen A.A., Antokhin A.M., Taranchenko V.F., Goncharov V.M., Aksenov A.V., Mitrofanov D.A., Berizovskaya E.I., Rodin I. A., Shpigun O.A. [Modern methods for the determination and identification of Goryanka flavonoids (epimedium)]. Moscow University Bulletin. 2. Chemistry, 2016, vol. 57, no. 3, pp. 172-183 (in Russian).
Dadáková E., Kalinova J. Determination of quercetin glycosides and free quercetin in buckwheat by capillary micellar electrokinetic chromatography. Journal of Separation Science, 2010, vol. 33, no. 11, pp. 1633–1638. doi: 10.1002/jssc.200900809.
Dmitrienko S.G., Stepanova A.V., Kudrinskaya V.A., Apyari V.V. Features of the separation of flavonoids by reverse phase high performance chromatography on a Luna 5u C18 column (2). Moscow University Bulletin. 2. Chemistry, 2012, vol. 53, no. 6, pp. 369-373 (in Russian).
Kuntić V., Pejić N., Ivković B., Vujić Z., Ilić K., Mićić S., Vukojević V. Isocratic RP-HPLC method for rutin determination in solid oral dosage forms. Journal of Pharmaceutical and Biomedical Analysis, 2007, vol. 43, no. 2, pp. 718-721. doi: 10.1016/j.jpba.2006.07.019.
Sumina E.G., Shtykov S.N., Sorokina O.N., Prozapas O.N., Uglanova V.Z. Liquid chromatography of some flavonoids on the reversed phase in aqueous-organic and modified micellar mobile phases. Journal of Analytical Chemistry, 2014, vol. 69, no. 12, pp.1179-11186. doi: 10.7868/S0044450214100156
Bulatov A.V., Falkova M.T., Pushina M.O., Moskvin L.N., Alekseeva G.M. Spectrophotometric determination of flavonoids in plant materials. Analitika i kontrol’ [Analytics and control], 2012, vol. 16, no. 4, pp. 358-362 (in Russian).
Veselova I., Malinina L., Barsukova M., Tokareva A., Buslova T., Sokolova L., Pirogov A., Shekhovtsova T. A novel multi-purpose enzymatic system and procedures for the rapid fluorescent determination of flavonoids in herbal pharmaceuticals and plant materials. Talanta. 2017, vol. 171, pp. 108–114. doi: 10.1016/j.talanta.2017.04.065
Gil E.S., Couto R.O. Flavonoid electrochemistry: a review on the electroanalytical applications. Revista Brasileira de Farmacognosia, 2013, vol. 23, no. 3, pp. 542-558. doi: 10.1590/S0102-695X2013005000031
Ziyatdinova G., Kozlova E., Budnikov H. Poly(gallic acid)/MWNT-modified electrode for the selective and sensitive voltammetric determination of quercetin in medicinal herbs. Journal of Electroanalytical Chemistry, 2018, vol. 821, pp. 73-81. doi:10.1016/j.jelechem.2017.12.071.
Stalikas C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. Journal of Separation Science, 2007, vol. 30, no. 18, pp. 3268-3295. doi 10.1002/jssc.200700261
Feng W., Hao Z., Li M. Isolation and Structure Identification of Flavonoids. In: Flavonoids, from biosynthesis to human health / Ed. by Justino G.C. Intech Open, 2017, pp. 17-43. doi: 10.5772/67810
Shil'ko E.A., Milevskaya V.V., Temerdashev Z.A., Kiseleva N.V. Solid-phase concentration of phenolic substances from aqueous extracts of medicinal plant raw materials on the example of Hypericum (Hypericum perforatum L.). Analitika i kontrol’ [Analytics and control], 2018, vol. 22, no. 3, pp. 303-314. doi: 10.15826/analitika.2018.22.3.013 (in Russian).
Tolmacheva V.V., Apyari V.V., Kochuk E.V., Dmitrienko S.G. Magnetic sorbents based on nanoparticles of iron oxides for the isolation and concentration of organic compounds. Journal of Analytical Chemistry, 2016, vol. 71, no. 4, pp. 321-338. doi: DOI: 10.1134/S1061934816040079
Pryazhnikov D.V., Kiseleva M.S., Kubrakova I.V. Magnetic surface-modified nanosized sorbent for MSPE-HPLC-UV determination of 4-nonylphenol in natural waters] Analitika i kontrol’ [Analytics and Control], 2015, vol. 19, no. 3, pp. 220-229. (in Russian)
Kozitsina A.N., Malysheva N.N., Utepova I.A., Glazyrina Y.A., Matern A.I., Brainina K.Z., Chupakhin O.N. An enzyme-free electrochemical method for the determination of E. coli using Fe3O4 nanocomposites with a SiO2 shell modified by ferrocene. Journal of Analytical Chemistry, 2015, vol. 70, no. pp. 540-545. DOI: 10.1134/S1061934815050068
Egunova O.R., Konstantinova T.A., Shtykov S.N. [Magnetic magnetite nanoparticles in separation and concentration] Izvestiia saratovskogo universiteta. Novaia seriia. Seriia Khimiia. Biologiia. Ekologiia, 2014, vol. 14, no. 4, pp. 27-34 (In Rissian).
Wu J., Xiao D., Zhao H., He H., Peng J., Wang C., Zhang C., He J. A nanocomposite consisting of graphene oxide and Fe3O4 magnetic nanoparticles for the extraction of flavonoids from tea, wine and urine samples. Microchimica Acta, 2015, vol. 13, pp. 2299-2306. doi:10.1007/s00604-015-1575-8.
He H., Yuan D., Gao Zh., Xiao D., He H., Dai H., Peng J., Li N. Mixed hemimicelles solid-phase extraction based on ionic liquid-coated Fe3O4/SiO2 nanoparticles for the determination of flavonoids in bio-matrix samples coupled with high performance liquid chromatography. Journal of Chromatography A, 2014, vol. 1324, pp. 78– 85.doi: 10.1016/j.chroma.2013.11.021.
Hu K, Qiao J, Wu X, Yang H, Huang Y, Zhang S Poly(calixarene ionic liquid) modified Fe3O4 nanoparticles as new sorbent for extraction of flavonoids in fruit juice and green tea. Microchemical Journal, 2018, vol. 143, pp. 39–46. doi:10.1016/j.microc.2018.07.029.
Liu Ch., Liao Y., Chen L., Li Y. Fabrication of N,N-dimethyldodecylamine functionalized magnetic adsorbent for efficient enrichment of flavonoids. Talanta, 2019, vol. 194, pp. 771-777. doi: j.talanta.2018.10.061.
Egunova O.R., Reshetnikova I.S., German S.V., Kazimirova K.O., Habibullin V.R., ZHelobickaya E.A., SHtykov S.N. [Sorption-fluorimetric determination of enrofloxacin using magnetite nanoparticles modified with polyethylenimine]. Izvestiia saratovskogo universiteta. Novaia seriia. Seriia Khimiia. Biologiia. Ekologiia, 2016, vol. 16, no. 1, pp. 48-52. doi:10.18500/1816-9775-2016-16-1-48-52 (In Russian)
Sodipo B.K., Aziz A.A. Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. Journal of Magnetism and Magnetic Materials, 2016, vol. 416, pp. 275–291. doi:10.1016/j.jmmm.2016.05.019.
Urusov A.E., Petrakova A.V., Zherdev A.V., Dzantiev B.B. [The use of magnetic nanoparticles in immunoassay]. Rossiiskie nanotekhnologii [Russian nanotechnology]. 2017, Vol. 12, no. 9-10, pp. 3-13.
Sumina E.G., Shtykov S.N., Sorokina O.N., Petrakova A.V., Uglanova V.Z. [Flavonoids thin layer chromatography on silica gel in modified micellar mobile phases based on sodium dodecyl sulfate]. Sorbtsionnye i khromatograficheskie protsessy [Sorption and chromatographic processes], 2014, vol. 14, no. 1, pp. 52-64 (In Russian).
Kazakova O.A., Gun'ko V.M., Lipkovskaya N.A., Voronin E.F., Pogorelyi V.K. Interaction of Quercetin with Highly Dispersed Silica in Aqueous Suspensions. Colloid Journal, 2002, vol. 64, no. 4. pp. 412-418.
Kurepa J., Nakabayashi R., Paunesku T., Suzuki M., Saito K., Woloschak G.E., Smalle J.A. Direct isolation of flavonoids from plants using ultra-small anatase TiO2 nanoparticles. Plant Journal, 2014, vol. 77, pp. 443–453. doi:10.1111/tpj.12361
Suh,J., Paik H-J., Hwang B.K. Ionization of Poly(ethylenimine) and Poly(allylamine) at Various pH's. Bioorganic Chemistry, 1994, vol. 22, no. 3. pp. 318-327. doi:10.1006/bioo.1994.1025
Ссылки
- На текущий момент ссылки отсутствуют.
